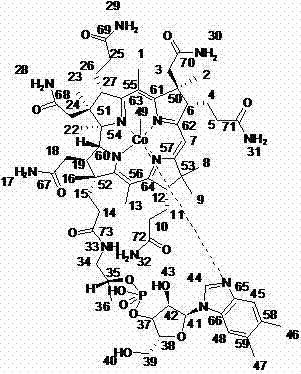
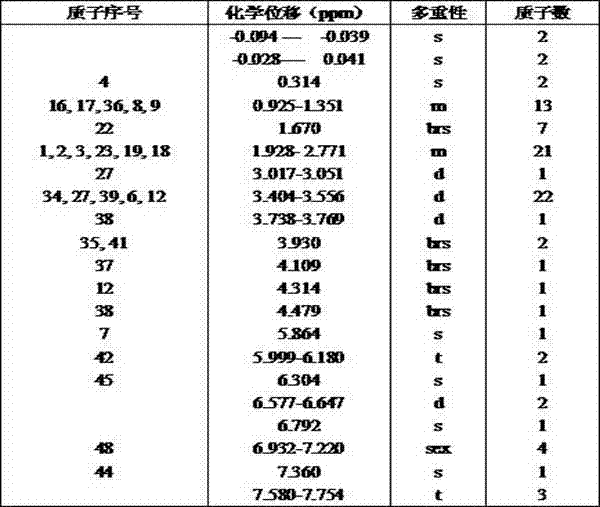
Novel chemical synthesis method for preparing mecobalamine
A kind of methylcobalamin, a new type of technology, applied in the field of chemical synthesis for synthesizing methylcobalamin, can solve the problems of serious environmental pollution, difficult to remove, purchase and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Reductive methylation
[0031] In a 500ml glass reaction bottle, add 20g cyanocobalamin, 1.4g cobalt chloride hexahydrate, 250ml purified water according to the proportion, stir and cool to 10-15°C to dissolve, keep stirring at 10-15°C, start to drop and dissolve NaBH at the same time 4 Purified aqueous solution (6g / 60ml), methanol solution of formaldehyde (37-40% w / w) aqueous solution (40.5ml formaldehyde aqueous solution / 25.5ml methanol), after about 45 minutes, continue to react for 2 hours. After the reaction, use 36 Adjust the pH of the reaction liquid to 6.0 with % acetic acid, stir again for 15 minutes until the pH remains unchanged, then control the vacuum degree to -0.08 MPa, and distill under reduced pressure at a temperature below 40°C.
[0032] 2), elution
[0033] Adsorb the reaction solution with a macroporous resin column (macroporous resin model: Amberlite XAD-2), and wash repeatedly with purified water to remove inorganic salts. Subsequently, 20% a...
Embodiment 2
[0040] 1) Reductive methylation
[0041] In a 500ml glass reaction bottle, add 20g cyanocobalamin, 1.4g cobalt chloride hexahydrate, 250ml purified water according to the proportion, stir and cool to 10-15°C to dissolve, keep stirring at 10-15°C, start to drop and dissolve NaBH at the same time 4 Purified aqueous solution (8g / 80ml), formaldehyde solution (37-40% w / w) in methanol solution (54ml formaldehyde solution / 34ml methanol), drop it in about 1 hour, continue to react for 2 hours, after the reaction, use 36% Adjust the pH of the reaction solution to 6 with acetic acid, stir again for 15 minutes until the pH remains unchanged, then control the vacuum to -0.08 MPa, and distill under reduced pressure at a temperature below 40°C.
[0042] 2), elution
[0043] Adsorb the reaction solution with a macroporous resin column (macroporous resin model: Amberlite XAD-2), and wash repeatedly with purified water to remove inorganic salts. Subsequently, 20% acetone was added for elutio...
Embodiment 3
[0050] 1) Reductive methylation
[0051] In a 500ml glass reaction bottle, add 20g cyanocobalamin, 1.4g cobalt chloride hexahydrate, 250ml purified water according to the proportion, stir and cool to 10-15°C to dissolve, keep stirring at 10-15°C, start to drop and dissolve NaBH at the same time 4 Purified aqueous solution (10g / 100ml), formaldehyde solution (37-40% w / w) in methanol solution (67.5ml formaldehyde solution / 42.5ml methanol), drop it in about 1.5 hours, continue to react for 2 hours, after the reaction, use Adjust the pH of the reaction solution to 6 with 36% acetic acid, stir for 15 minutes and retest until the pH remains unchanged, then control the vacuum degree to -0.08 MPa, and distill under reduced pressure at a temperature below 40°C. .
[0052] 2), elution
[0053] Adsorb the reaction solution with a macroporous resin column (macroporous resin model: Amberlite XAD-2), and wash repeatedly with purified water to remove inorganic salts. Subsequently, 20% acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com