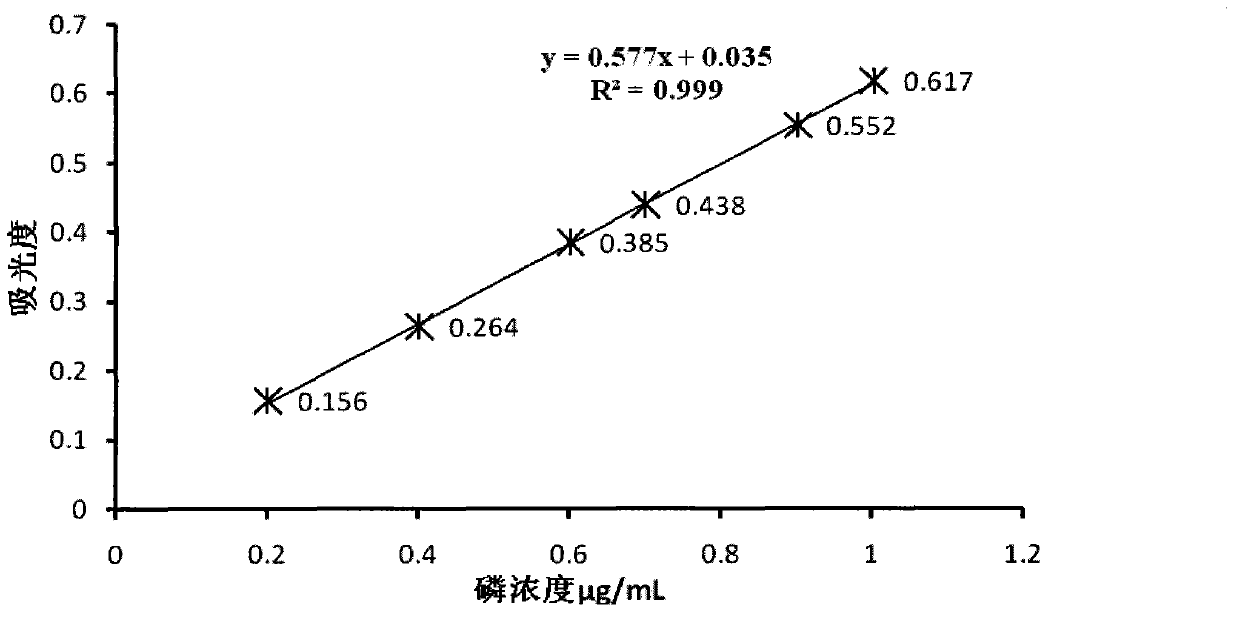
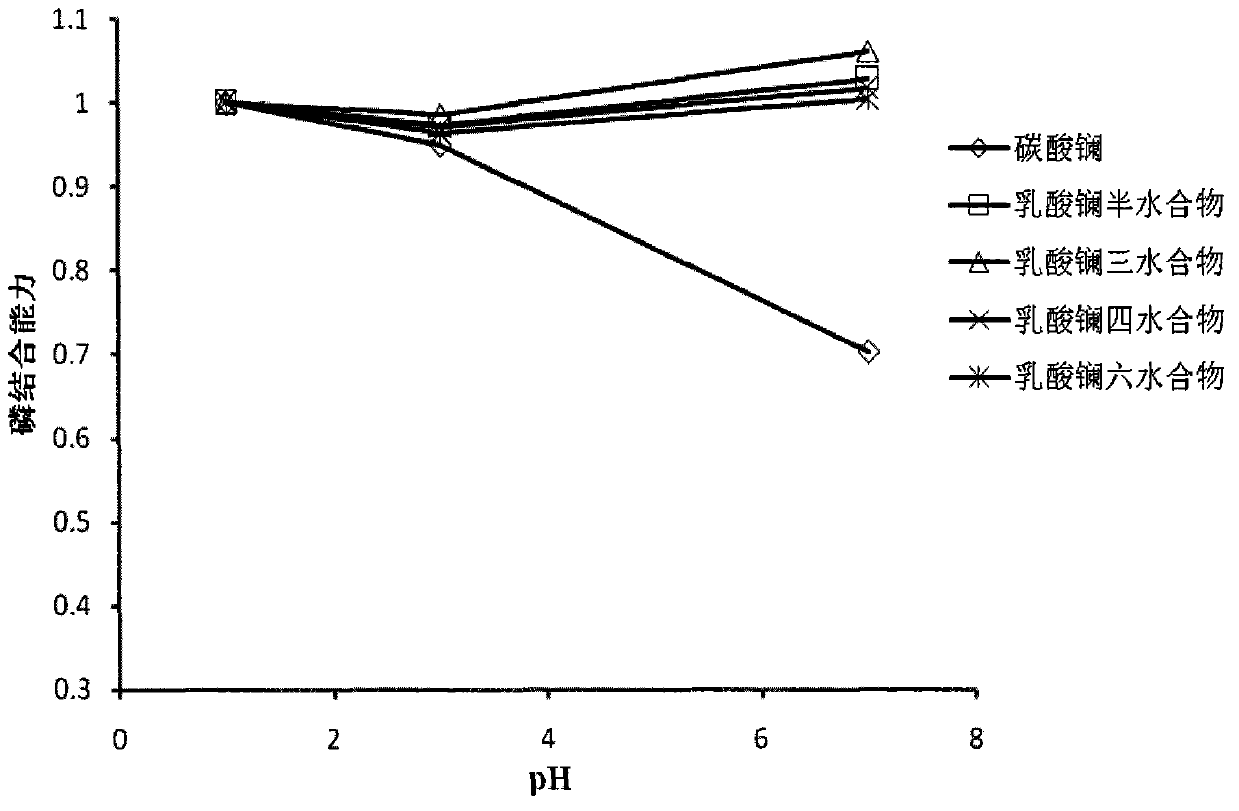
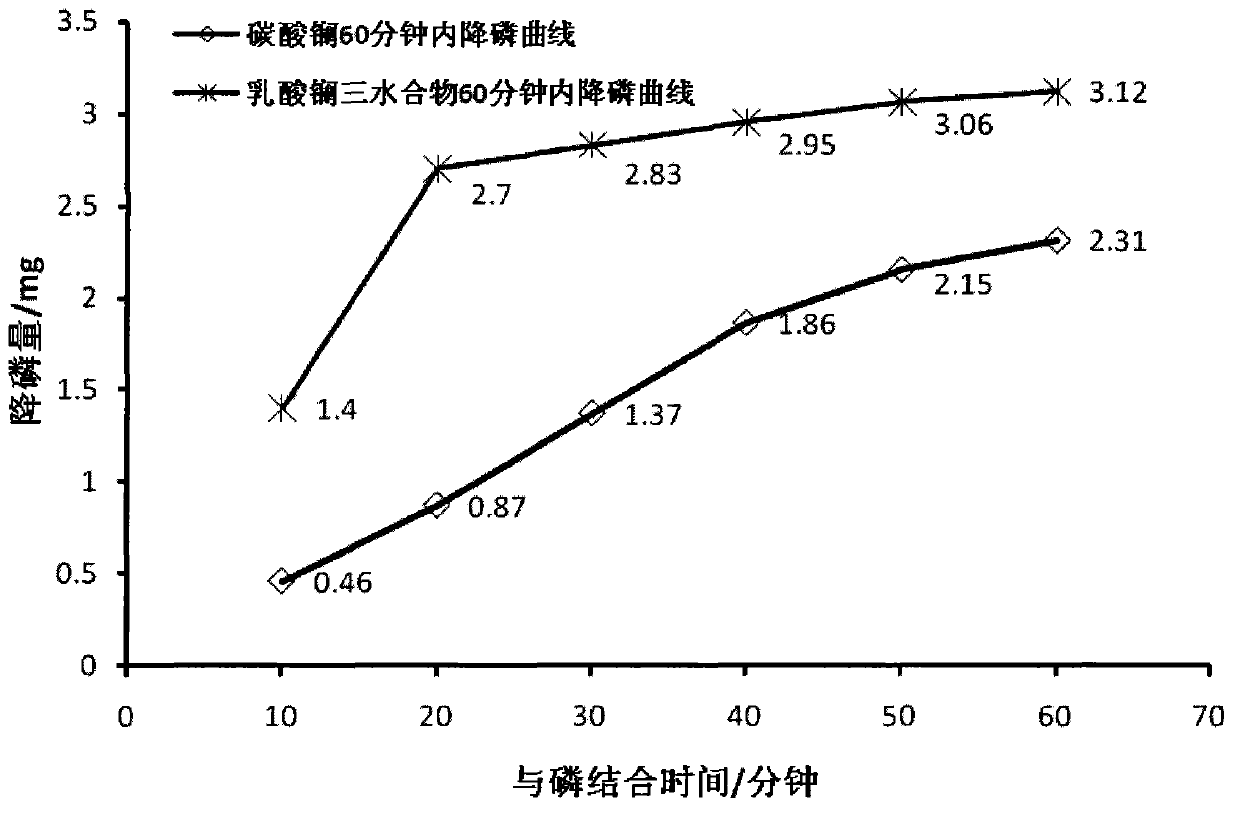
Stable and efficient dephosphorization composition
A composition and high-efficiency technology, applied in the direction of drug combination, active ingredients of anhydride/acid/halide, metabolic diseases, etc., can solve the problems of limitation, phosphorus reduction ability, pH environment change, etc., and achieve reduced dosage and comprehensive utilization efficiency High, less involved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0051] The present embodiment illustrates the preparation method of lanthanum lactate trihydrate of the present invention, and concrete steps are as follows:
[0052] a) Weigh 1.0 kg of lanthanum carbonate and fully pulverize it through an 80-mesh sieve, and evenly disperse it in an appropriate amount of water at 65-70°C;
[0053] b) Add 0.9kg of lactic acid to the above a), mix evenly under stirring conditions, and react completely under airtight conditions at 65-70°C;
[0054] c) Evaporate and dry the above obtained solution at 75-80°C to constant weight, dissolve it in 10.0L of water and filter, take the filtrate and evaporate and dry it to constant weight at 75-85°C to obtain crude lanthanum lactate;
[0055] d) The crude lanthanum lactate obtained above was washed with 5.0 L of water, concentrated, air-dried and pulverized, and then vacuum-dried at 90-100°C for 7.5 hours to obtain lanthanum lactate trihydrate, weighing 1.72 kg;
preparation Embodiment 2
[0057] This embodiment illustrates the preparation method of lanthanum lactate hydrate containing different crystal water structures according to the present invention, and the specific steps are as follows:
[0058] a) Weigh 1.2kg of lanthanum carbonate and fully pulverize it through an 80-mesh sieve, and evenly disperse it in an appropriate amount of water at 65-70°C;
[0059] b) Add 1.3kg of lactic acid to the above a), mix evenly under stirring conditions, and react completely under airtight conditions at 65-70°C;
[0060] c) Evaporate and dry the above obtained solution at 75-80°C to constant weight, dissolve it with 12.0L of water and filter, take the filtrate and evaporate and dry it at 75-85°C to constant weight to obtain crude lanthanum lactate;
[0061] d) The crude lanthanum lactate obtained above was washed with 10.0 L of water, concentrated, blast-dried, pulverized, and then vacuum-dried at 100-110°C for 5.5 hours to obtain lanthanum lactate tetrahydrate, weighing...
preparation Embodiment 3
[0067] The preparation of the stable and high-efficiency phosphorus-reducing composition tablet of the present invention:
[0068] Prescription composition:
[0069]
[0070] Preparation process: 1) After pulverizing an appropriate amount of lanthanum lactate hydrate, microcrystalline cellulose, lactose, crospovidone, and sodium carboxymethyl starch through an 80-mesh sieve, weigh the recipe quantity for later use; 2) Prepare 3.0% hydroxy The propyl methylcellulose solution is ready for use; 3) Put the weighed components in 1) into the mixer and mix them evenly; 4) Add the prescription amount 2) to the mixture to make soft materials, and granulate them with a high-efficiency wet granulator , dried below 60°C; 5) pass the dried granules through a 16-mesh sieve for granulation, add magnesium stearate, mix well, and then compress on a tablet machine to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com