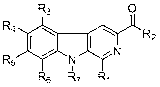
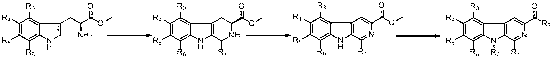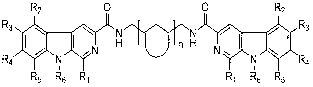Substituted beta-carboline compound and preparation method thereof
A compound, carboline technology, applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of multi-drug resistance, easy mutation, and fast growth of tumor cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] .
[0063] 1) Synthetic compound 9
[0064] In a 1L round bottom flask, dissolve L-tryptophan methyl ester hydrochloride (100 g, 0.45 mol) in 500 mL of acetonitrile and 50 mL of methanol, add acetaldehyde (40 g, 0.9 mol), and react at 80 °C Under stirring for 16 h, the solution was spin-dried under vacuum to obtain the crude product 9 (109 g, 88% yield). ESI-MS: 244.7 [M+H] + .
[0065] 2) Synthetic compound 10
[0066] In a 500 mL round bottom flask, the compound 9 (86 g, 0.35 mol) was dissolved in 100 mL DMF, and potassium permanganate (113 g, 0.70 mol) was added at 0°C, and the reaction was stirred at 30°C for 3 h. After the raw materials were consumed, the solid was removed by filtration. After the solution was evaporated to dryness, the compound was obtained 10 (66 g, 78% yield). ESI-MS: 240.9 [M+H] + , 1 H NMR (CD 3 OD, 400 MHz) d 8.60 (s, 1H), 8.12-8.09 (m, 1H), 7.55-7.53 (m, 2H), 7.28-7.24 (m, 1H), 3.972 (s, 3H), 2.77 (s, 3H).
[0067] 3...
Embodiment 2
[0072] .
[0073] 1) Synthetic compounds 12
[0074] Compound in 250 mL round bottom flask 10 (6 g, 25 mmol) was dissolved in 100 mL tetrahydrofuran, 60% sodium hydrogen (2.4 g, 100 mmol) was added at 0°C, stirred for 1 h, and methyl iodide (7.1 g, 50 mmol) was added, and the reaction solution was heated at room temperature After stirring overnight, neutralize with concentrated hydrochloric acid, obtain a yellow precipitate after filtration, and obtain a yellow powder compound after drying the precipitate 12 (5.6 g, 93% yield).
[0075] 2) Synthetic compounds 2
[0076] In a 10 mL reaction vial, compound 12 (70 mg) was dissolved in THF (2 mL), added 1 equivalent of 1,1`-carbonyldiimidazole (CDI) and stirred for 1 h, then added 1 equivalent of piperazine, and the reaction solution was stirred at room temperature for 16 h. Preparative high performance liquid phase purification to obtain the compound 2 (50 mg, 56% yield). ESI-MS: 308.4 [M + H] + ; 1 H NMR (DM...
Embodiment 3
[0078] .
[0079] 1) Synthetic compounds 13
[0080] Compound in 250 mL round bottom flask 10 (5 g, 20.8 mmol) was dissolved in 100 mL of tetrahydrofuran, 60% sodium hydrogen (2 g, 83 mmol) was added at 0°C, and after stirring for 1 h, n-butyl bromide (4.25 g, 31.2 mmol) was added, and the reaction solution was After stirring overnight at room temperature, neutralize with concentrated hydrochloric acid, obtain a yellow precipitate after filtration, and obtain a light yellow powder compound after drying the precipitate 13 (5.5 g, 95% yield). ESI-MS: 282.9 [M+H] + , 1 H NMR (DMSO-d6, 400 MHz) d 8.96 (s, 1H), 8.48 (d, J=8 Hz, 1H), 8.85 (d, J=8 Hz, 1H), 7.71 (t, J=8Hz, 1H), 7.39 (t, J=8Hz, 1H), 4.66 (t, J=8Hz, 2H), 3.150 (s, 3H), 1.77-1.73 (m, 2H), 1.40-1.34 (m, 2H), 0.90(t, J= 7.2 Hz, 3H) .
[0081] 2) Synthetic compounds 3
[0082] In a 10 mL reaction vial, compound 13(70 mg) was dissolved in THF (2 mL), and 1.2 equivalents of N-methylpiperazine, 2 equival...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com