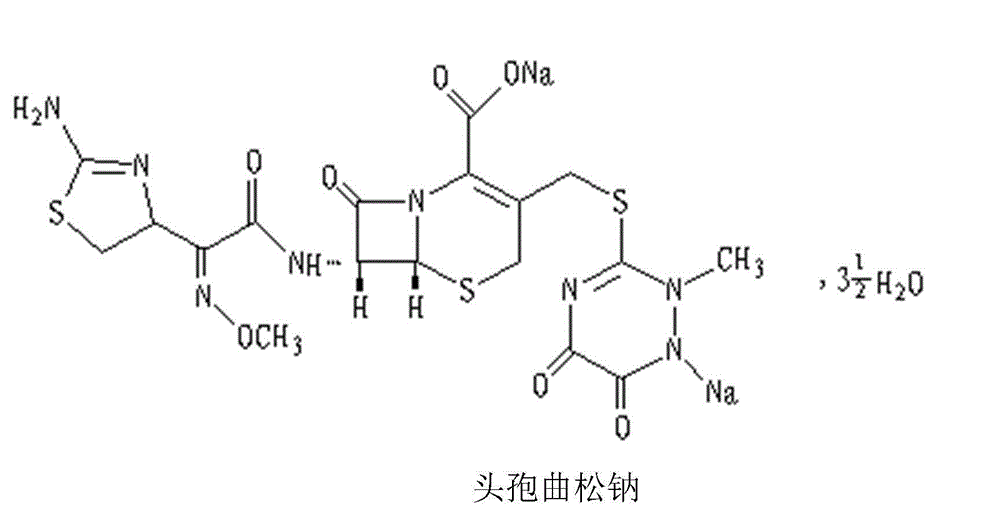
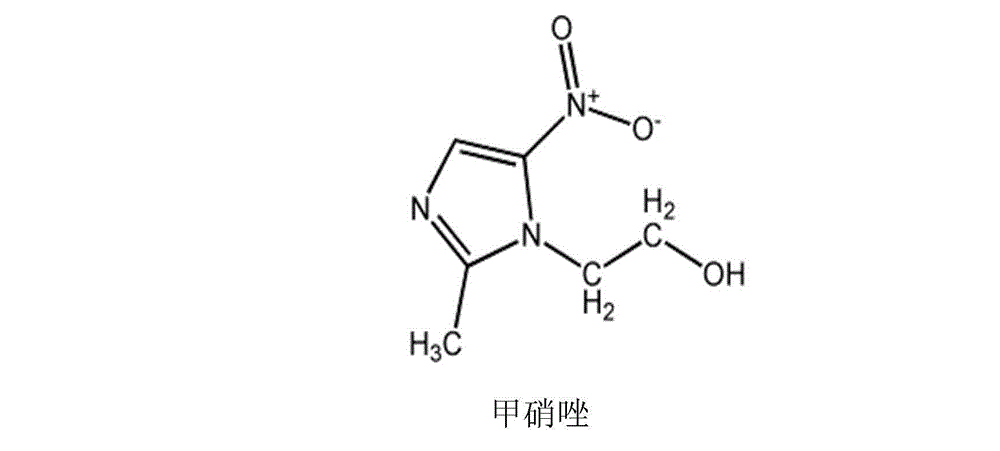
Ceftriaxone sodium pharmaceutical composition and preparation method thereof
A technology of ceftriaxone sodium and a composition, which is applied in the field of pharmaceutical preparations and preparations, can solve the problems of strong toxic and side effects of metronidazole, large dosage of metronidazole, etc., and achieves the effects of simple preparation process and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one, ceftriaxone sodium composition powder for injection, in 1000 pieces
[0034] Prescription (specification: 0.5g / bottle):
[0035]
[0036] Preparation Process:
[0037] Add the prescribed amount of metronidazole lipid microspheres (calculated as metronidazole) directly to an appropriate amount of water for injection; stir and dissolve, then add a pH regulator to adjust the pH of the solution so that it is in the range of 6.5-6.8; 0.45μm and 0.22μm filter membranes are used for sterilization cycle filtration for 30 minutes; after the sampling inspection meets the requirements, the solution is divided into stainless steel trays, put into a freeze-drying box, and freeze-dried to obtain sterile freeze-dried metronidazole powder, pulverized and sieved; after mixing the pulverized and sieved metronidazole freeze-dried powder and ceftriaxone sodium sterile powder, after the sampling inspection meets the requirements, subpackage according to the marked amount...
Embodiment 2
[0038] Embodiment two, ceftriaxone sodium composition powder injection for injection, in 1000 pieces
[0039] Prescription (specification: 1.0g / bottle):
[0040]
[0041] Preparation Process:
[0042] Add the prescribed amount of metronidazole lipid microspheres (calculated as metronidazole) directly to an appropriate amount of water for injection; stir and dissolve, then add a pH regulator to adjust the pH of the solution so that it is in the range of 6.5-6.8; 0.45μm and 0.22μm filter membranes are used for sterilization cycle filtration for 30 minutes; after the sampling inspection meets the requirements, the solution is divided into stainless steel trays, put into a freeze-drying box, and freeze-dried to obtain sterile freeze-dried metronidazole powder, pulverized and sieved; after mixing the pulverized and sieved metronidazole freeze-dried powder and ceftriaxone sodium sterile powder, after the sampling inspection meets the requirements, subpackage according to the mar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com