CuO-CeO2/MWCNT(Multi Walled Carbon Nanotubes) catalyst and preparation method thereof
A technology of catalysts and composite oxides, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, metal/metal oxides/metal hydroxide catalysts, etc. Catalyst is expensive and other problems, to achieve the effect of simple preparation method, high activity and stability, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
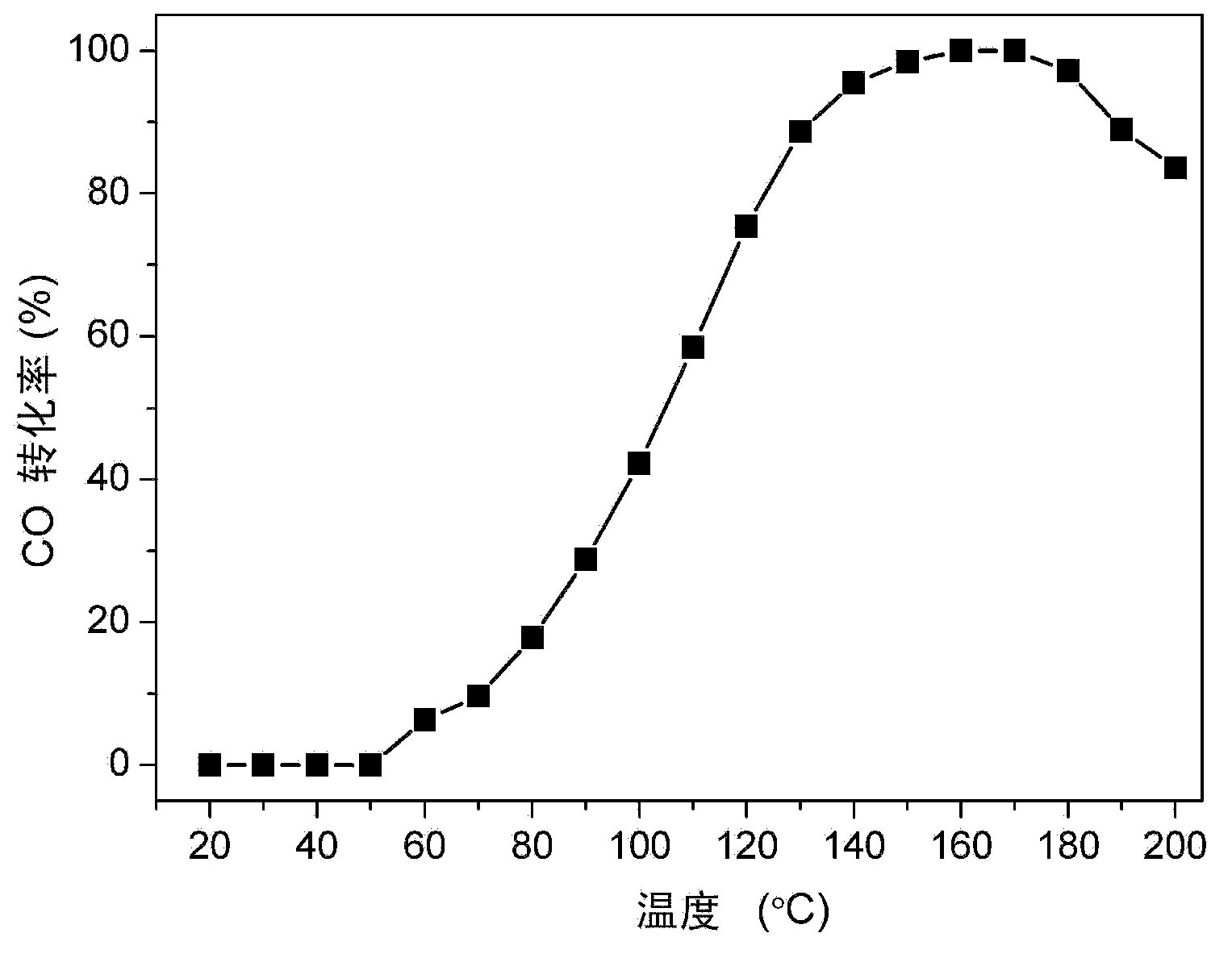
[0039] Preparation Example 1: Preparation of CuO-CeO of the present invention 2 / MWCNT catalyst
[0040] Put 2 g of multi-walled carbon nanotubes of different diameters (produced by Chengdu Organic Chemistry Co., Ltd., Chinese Academy of Sciences) into a 250ml round bottom flask, add 37% to 65% of HNO 3 100ml, put it into an oil bath and reflux at 110-140°C for 5 hours, naturally cool to room temperature, filter and wash until pH=7, put it in an oven and dry at 110°C for 10 hours.
[0041] Weigh a certain amount of Cu(NO 3 ) 2 and Ce(NO 3 ) 3 Put into a 100ml beaker, add 30ml of acetone solvent and magnetically stir for 5 minutes, then weigh 0.3g of acid-pretreated carbon nanotubes and add them to the acetone solution (the metal oxide accounts for 20% of the total weight of the catalyst), and continue to stir for 5 minutes. Seal the mouth of the beaker with tin foil and put it into an ultrasonic machine for 3 hours of ultrasonication. The ultrasonic sample was stirred at ...
preparation example 2
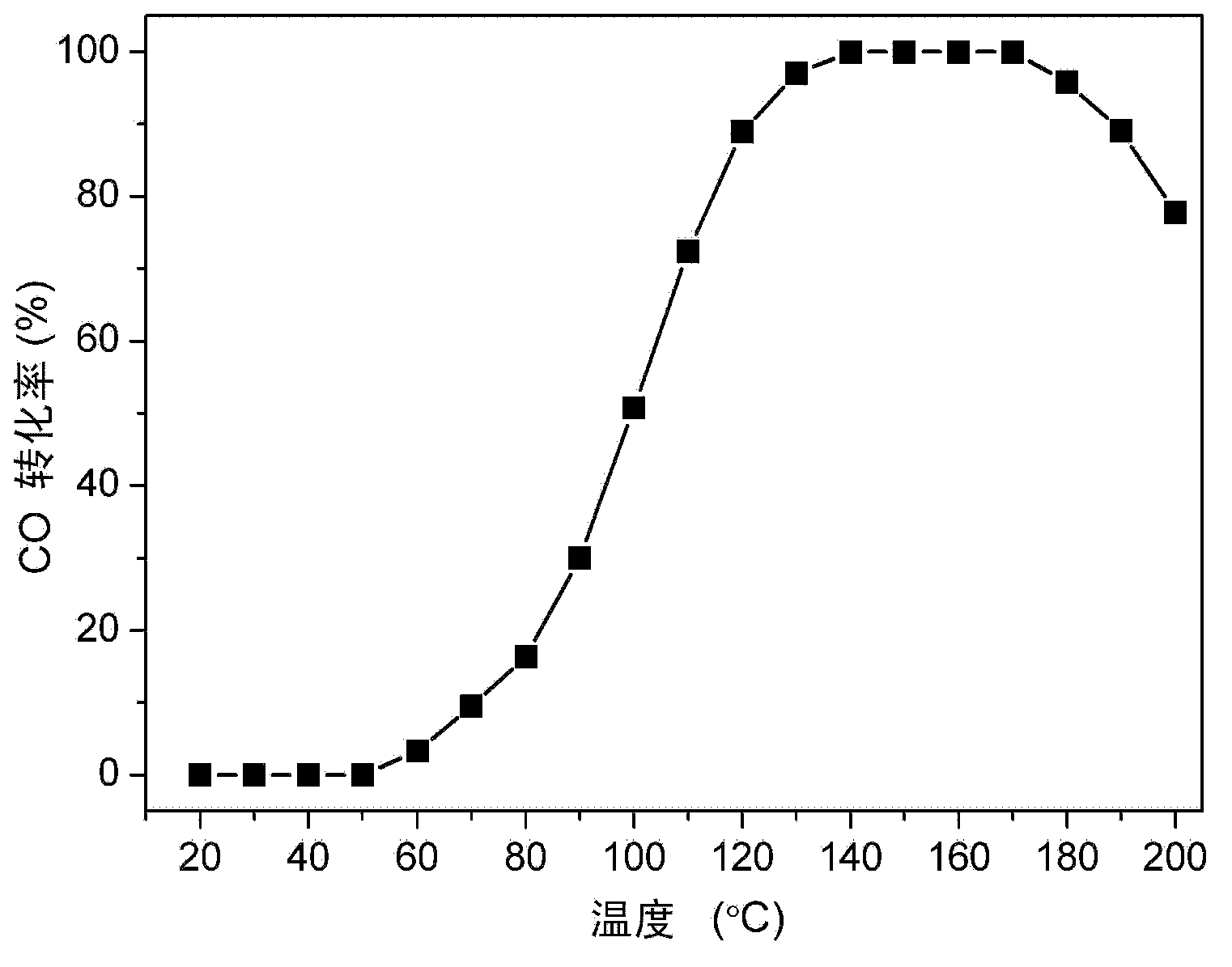
[0042] Preparation example 2: prepare activated carbon (AC) and aluminum oxide (Al 2 o 3 ) supported CuO-CeO 2 catalyst
[0043] Weigh a certain amount of Cu(NO 3 ) 2 and Ce(NO 3 ) 3 Put it into a 100ml beaker, add 30ml of acetone magnet and stir for 5 minutes, then weigh 0.3g of activated carbon or alumina and add it to the acetone solution (the metal oxide accounts for 20% of the total weight of the catalyst), continue to stir for 5 minutes, cover the mouth of the beaker with tin foil Seal it and place it in an ultrasonic machine for 3 hours of ultrasonication. The ultrasonicated sample is stirred at room temperature until it is dry, then placed in a constant temperature blast drying oven at 110°C for 12 hours, cooled naturally to room temperature, placed in a mortar and ground, and then placed in a tube furnace in high-purity N 2 Calcined at 350°C for 2 hours in the atmosphere, the heating rate was 2°C / min, and the final catalysts were 20wt% (0.5)CuO-(0.5)CeO 2 -(35...
preparation example 3
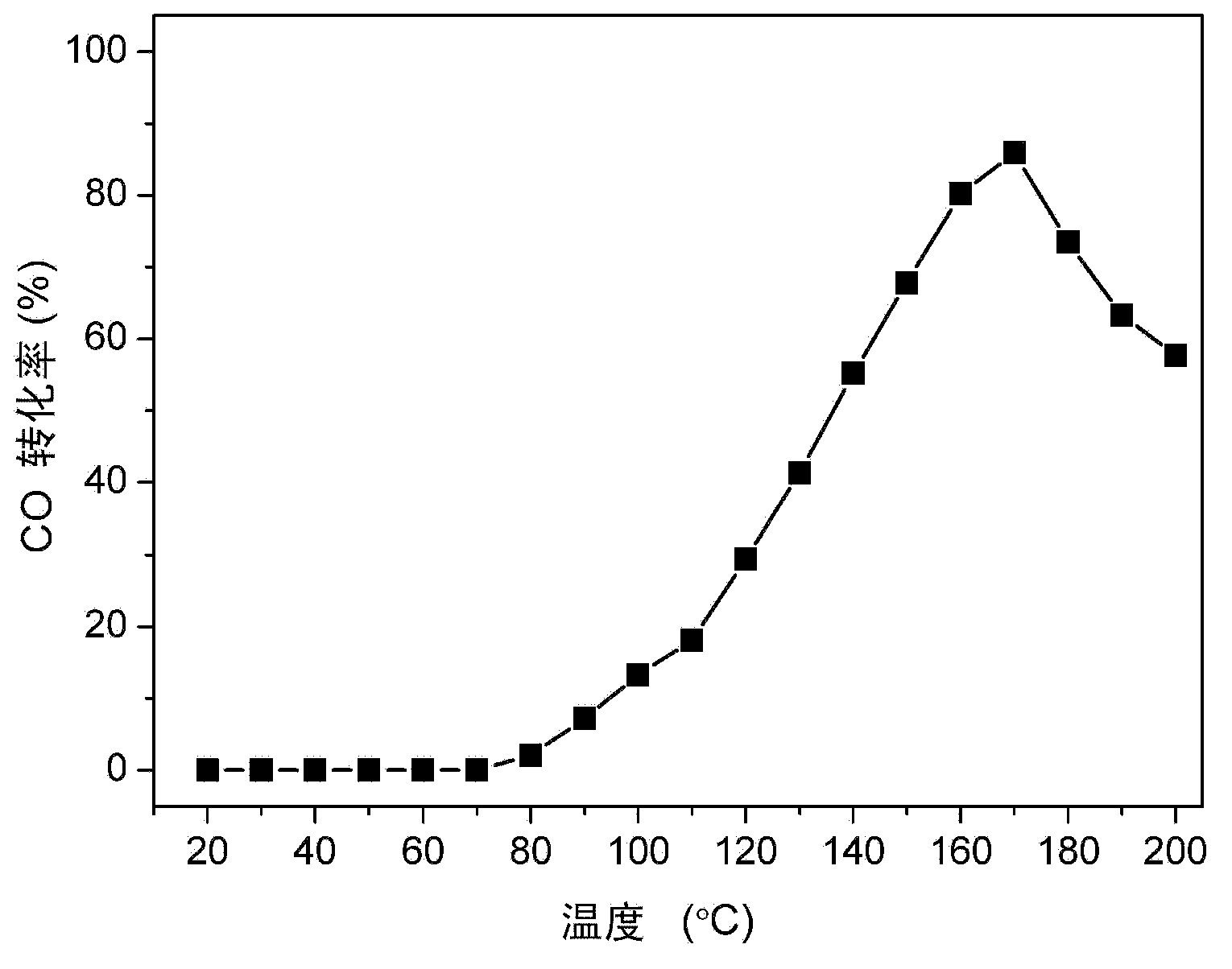
[0044] Preparation Example 3: Preparation of Unsupported CuO-CeO 2 catalyst
[0045] Weigh a certain amount of Cu(NO 3 ) 2 and Ce(NO 3 ) 3 Put it into a 100ml beaker, add 30ml of acetone and stir for 5 minutes, seal the mouth of the beaker with tin foil and put it in an ultrasonic machine for 3 hours. After ultrasonication, stir the sample until dry at room temperature, and then put it in a constant temperature blast drying oven Dry at 110°C for 12 hours, cool to room temperature naturally, put it into a mortar and grind it finely, then put it into a tube furnace and calcinate at 350°C for 2 hours in a high-purity N2 atmosphere, the heating rate is 2°C / min, and finally the obtained The catalyst is unsupported (0.5)CuO-(0.5)CeO 2 -(350).
[0046] (2) Application Examples and Comparative Examples
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com