A kind of Fe2O3 catalyst with tungsten oxide surface modification, preparation method and application thereof
A technology of surface modification and catalyst, applied in the field of Fe2O3 catalyst, preparation and application of surface modification of tungsten oxide, to achieve good NOx purification efficiency, catalytic activity and N2 generation selectivity improvement, and the effect of suppressing non-selective oxidation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
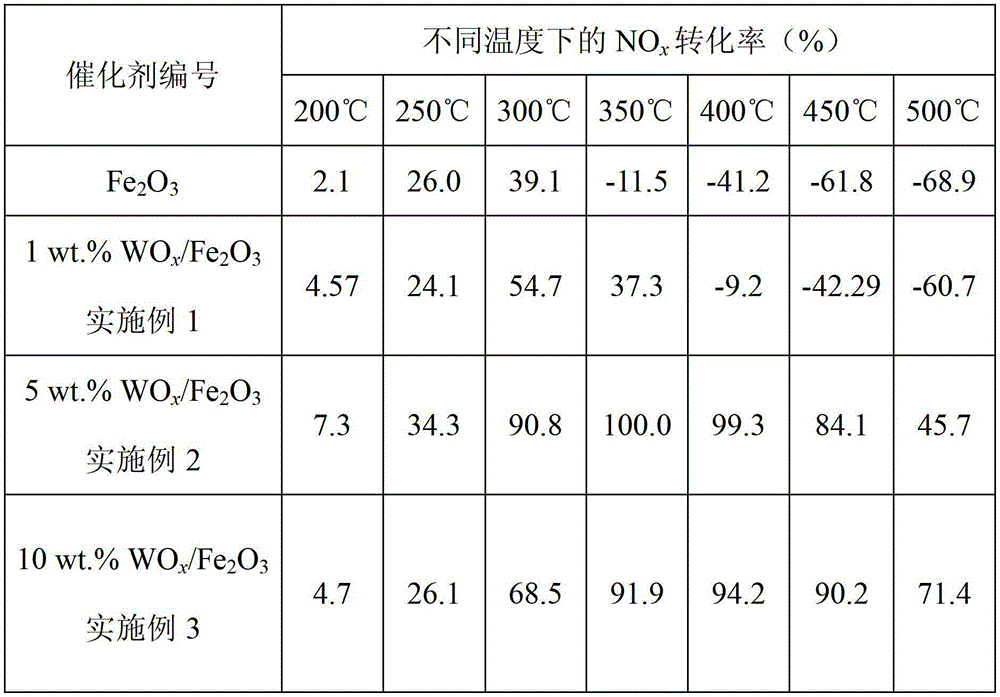
[0045] A kind of Fe modified by tungsten oxide surface 2 o 3 Catalyst, described catalyst has following chemical composition: WO x / Fe 2 o 3 , x=2~3, the WO x The mass percent of the catalyst is 1wt.%.
[0046] A kind of Fe modified by tungsten oxide surface 2 o 3 The preparation method of catalyst, described method comprises the steps:
[0047] (1) Prepare ferric nitrate solution, add excess urea precipitant to the solution, the molar ratio of urea / iron source is 10:1, stir continuously for 12 hours in a water bath at 90°C to completely precipitate iron ions, and filter the precipitate and washed, and then the filter cake was dried in an oven at 100°C for 5h, and finally calcined in a muffle furnace at 500°C for 3h in an air atmosphere to obtain Fe 2 o 3 ;
[0048] (2) In the presence of oxalic acid, ammonium tungstate solution was prepared to control WO x Accounting for the mass percent of the catalyst is 1wt.%, adding Fe to the ammonium tungstate solution 2 o 3...
Embodiment 2
[0050] A kind of Fe modified by tungsten oxide surface 2 o 3 Catalyst, described catalyst has following chemical composition: WO x / Fe 2 o 3 , x=2~3, the WO x The mass percent of the catalyst is 5wt.%.
[0051] A kind of Fe modified by tungsten oxide surface 2 o 3 The preparation method of catalyst, described method comprises the steps:
[0052] (1) Prepare ferric nitrate solution, add excess urea precipitant to the solution, the molar ratio of urea / iron source is 10:1, stir continuously for 12 hours in a water bath at 90°C to completely precipitate iron ions, and filter the precipitate and washed, and then the filter cake was dried in an oven at 100°C for 5h, and finally calcined in a muffle furnace at 500°C for 3h in an air atmosphere to obtain Fe 2 o 3 ;
[0053] (2) In the presence of oxalic acid, ammonium tungstate solution was prepared to control WO x Accounting for the mass percent of the catalyst is 5wt.%, adding Fe to the ammonium tungstate solution 2 o 3...
Embodiment 3
[0055] A kind of Fe modified by tungsten oxide surface 2 o 3 Catalyst, described catalyst has following chemical composition: WO x / Fe 2 o 3 , x=2~3, the WO x The mass percent of the catalyst is 10wt.%.
[0056] A kind of Fe modified by tungsten oxide surface 2 o 3 The preparation method of catalyst, described method comprises the steps:
[0057] (1) Prepare ferric nitrate solution, add excess urea precipitant to the solution, the molar ratio of urea / iron source is 10:1, stir continuously for 12 hours in a water bath at 90°C to completely precipitate iron ions, and filter the precipitate and washed, and then the filter cake was dried in an oven at 100°C for 5h, and finally calcined in a muffle furnace at 500°C for 3h in an air atmosphere to obtain Fe 2 o 3 ;
[0058] (2) In the presence of oxalic acid, ammonium tungstate solution was prepared to control WO x Accounting for the mass percent of the catalyst is 10wt.%, adding Fe to the ammonium tungstate solution 2 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com