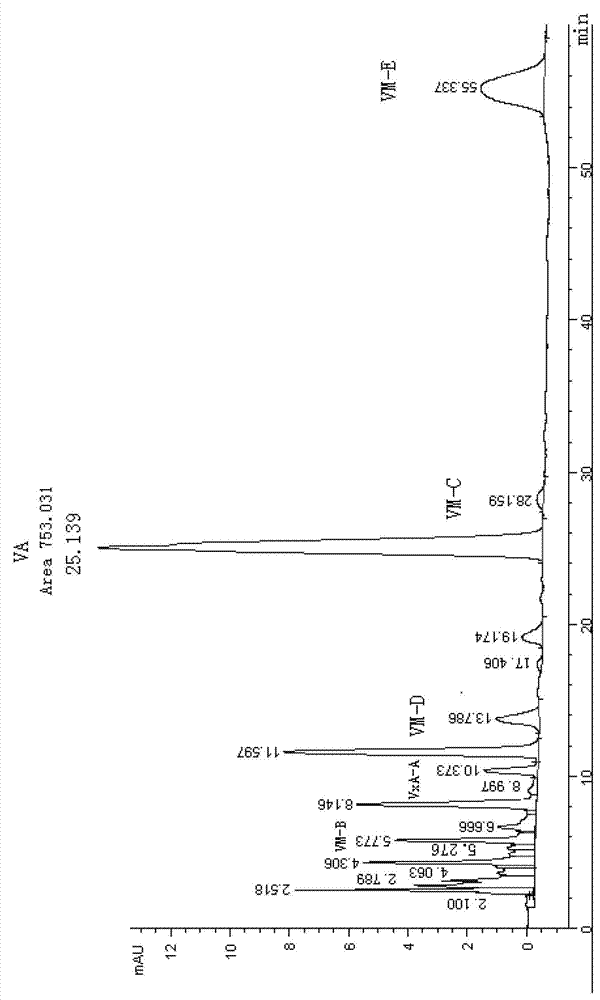
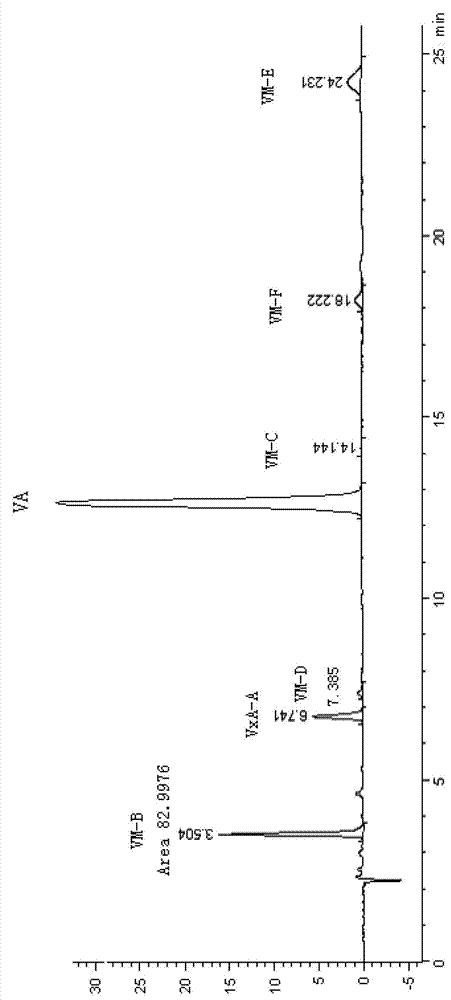
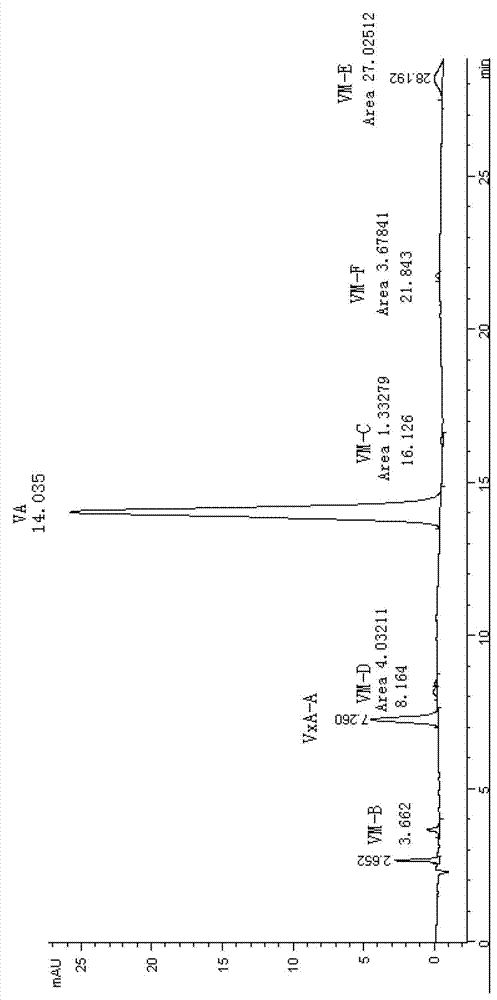
Qualitative and quantitative detection method for each component of validamycin
A quantitative detection method, the technology of Jinggangmycin, which is applied in the direction of measuring devices, material separation, and analysis materials, etc., can solve the problems of easily damaged chromatographic column structure, inability to separate and quantify components, and poor peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] A qualitative and quantitative detection method of Jinggangmycin A and related components, the steps are as follows:
[0081] The first step of mobile phase preparation: weigh 435.0mg of anhydrous K 2 HPO 4 In a 1000mL volumetric flask, add double distilled water to dissolve, dilute to the mark with double distillation, and shake well. Adjust the pH to 7.0 with phosphoric acid for later use, and filter through a 0.45 μm microporous membrane before use. Double distilled water was filtered through a 0.45 μm microporous membrane.
[0082] The second step of chromatographic instrument preparation: Install the YMC-PACK ODS-AQ chromatographic column, first equilibrate the chromatographic column with HPLC grade methanol at a flow rate of 1.0ml / min for about 40 minutes, and replace it with methanol after the baseline of the instrument is stable: twice distilled water = 3:97 (volume ratio), 1.0ml / min flow rate to wash the chromatographic column for about 40 minutes, after the...
Embodiment 2
[0091] Prepare the mobile phase, set the instrument parameters, equilibrate the chromatographic column, and compile the sample injection schedule as in Example 1.
[0092] Preparation of Jinggangmycin standard solution: Weigh 107.88mg of Jinggangmycin standard sample (purchased from Mitsui Corporation, VALIDAMYCIN STANDARD, Purity 93.8%, LOT.NO.04①) into a 50mL volumetric flask, add 2mL of 0.05mol / L H 2 SO 4 , double-distilled water to volume, then pipet 4, 5, and 6 mL to three 50-mL volumetric flasks respectively, and double-distilled water to volume, standard solution I Jinggangmycin A concentration is 161.9063 μg / ml, standard solution II The concentration of A is 202.3829 μg / ml, and the concentration of Jinggangmycin A in standard solution III is 242.8595 μgml.
[0093] Preparation of Jinggangmycin sample solution: Weigh 1385.93mg and 1415.25mg of high-purity Jinggangmycin analysis solution (pretreatment sample for producing high-purity Jinggang original drug) with batch n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com