Application of superparamagnetic iron oxide nanoparticle applied in transdermal drug delivery system
A technology of iron oxide nanoparticles and superparamagnetism, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, to achieve the effect of overcoming the single administration method and non-toxic skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Co-precipitation method to synthesize superparamagnetic iron oxide nanoparticles
[0019] Weigh 10mg ferric chloride (FeCl 2 4H 2 O) and 28mg sodium metasilicate (Na 2 SiO 3 9H 2 O) Dissolve in 30ml of degassed ultrapure water to obtain a mixed iron salt solution, adjust the pH value to 3.0 with hydrochloric acid solution, raise the temperature to 30°C, add lye drop by drop until the pH value of the reaction solution is 9-10 , stop adding lye, and then heat in a water bath, mature the reaction suspension at 80°C for 1 hour, centrifuge at 8000rpm for 5 minutes, separate the precipitate, wash the precipitate three times with ultrapure water and absolute ethanol, and place it at 70 °C, vacuum-dried and set aside.
[0020] (2) Surface amino modification
[0021] Ultrasonically disperse the precipitate obtained in step (1) in 100ml of boric acid solution with a pH of 5.0, add 2mg of carbodiimide for catalysis and 2ml of triethoxysilane, 80°C water bath, magnetic st...
Embodiment 2
[0025] (1) Magnetic fluid particle size and potential measurement
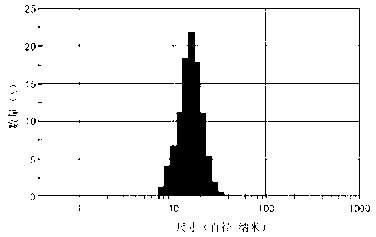
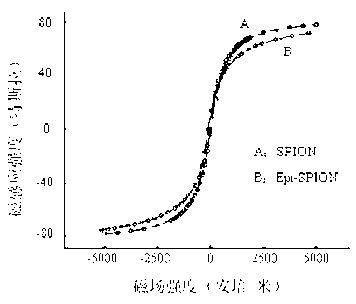
[0026] Use a 200-mesh copper mesh support film dedicated to transmission electron microscopy to dip an appropriate amount of the Epi-SPION suspension prepared in Example 1, dry it at room temperature, and observe the size and shape of the nanoparticles under a JEM-1200EX transmission electron microscope. For the results, see figure 1 , the particle size of Epi-SPION nanoparticles is 8-10nm, the particles are regular, approximately spherical, the dispersion is good, and no obvious agglomeration occurs; take another appropriate amount (about 0.5ml) of nanoparticle suspension, and ultrasonically After dispersion treatment, dilute to 3-5 times, and use Zetasizer Nano S90 high-sensitivity nanometer particle size analyzer to measure the particle size and Zeta potential of nanoparticles. For the measurement results, see figure 1 : The particle size of EPI-SPION is mainly distributed at 28.1nm, showing a normal distri...
Embodiment 3
[0030] (1) Cell culture
[0031] HaCaT cells (normal human skin cell line, KGI Biotechnology) were revived and incubated with RPM1640 medium containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured in an environment with EDTA-trypsinization and subcultured every other day.
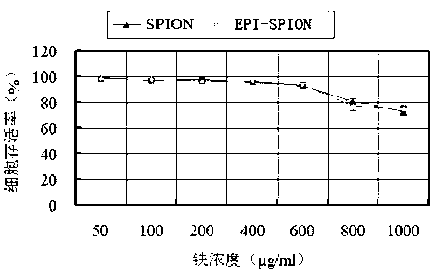
[0032] (2) Cytotoxicity study
[0033]The above-mentioned cells were subcultured to reach 80-90% confluence, digested with 0.25% trypsin-EDTA solution, and prepared into a single-cell suspension with RPM1640 culture medium containing 10% fetal bovine serum. 3 Inoculate / well in a 96-well plate; at 37°C, 5% CO 2 Cultivate under conditions for 24 hours; discard the supernatant, wash twice with PBS solution, add EPI-SPION, SPION-containing media in turn, the concentrations of nanoparticles in the media are 50 μg / ml, 100 μg / ml, 200 μg / ml, respectively. ml, 400μg / ml, 600μg / ml, 800μg / ml, 1000μg / ml of the above nanoparticle suspension and 10% fetal bovine serum culture solution 200μl, design 3 replicate well...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com