Multiphase microfluidic immunoblotting chip, and preparation method and application thereof
A technology of immunoblotting and microfluidics, which is applied in the fields of biotechnology and tissue engineering, can solve the problems of difficult biological laboratory use, high cost, and unsatisfactory detection, and achieve the effects of reducing costs, saving time, and improving effects and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
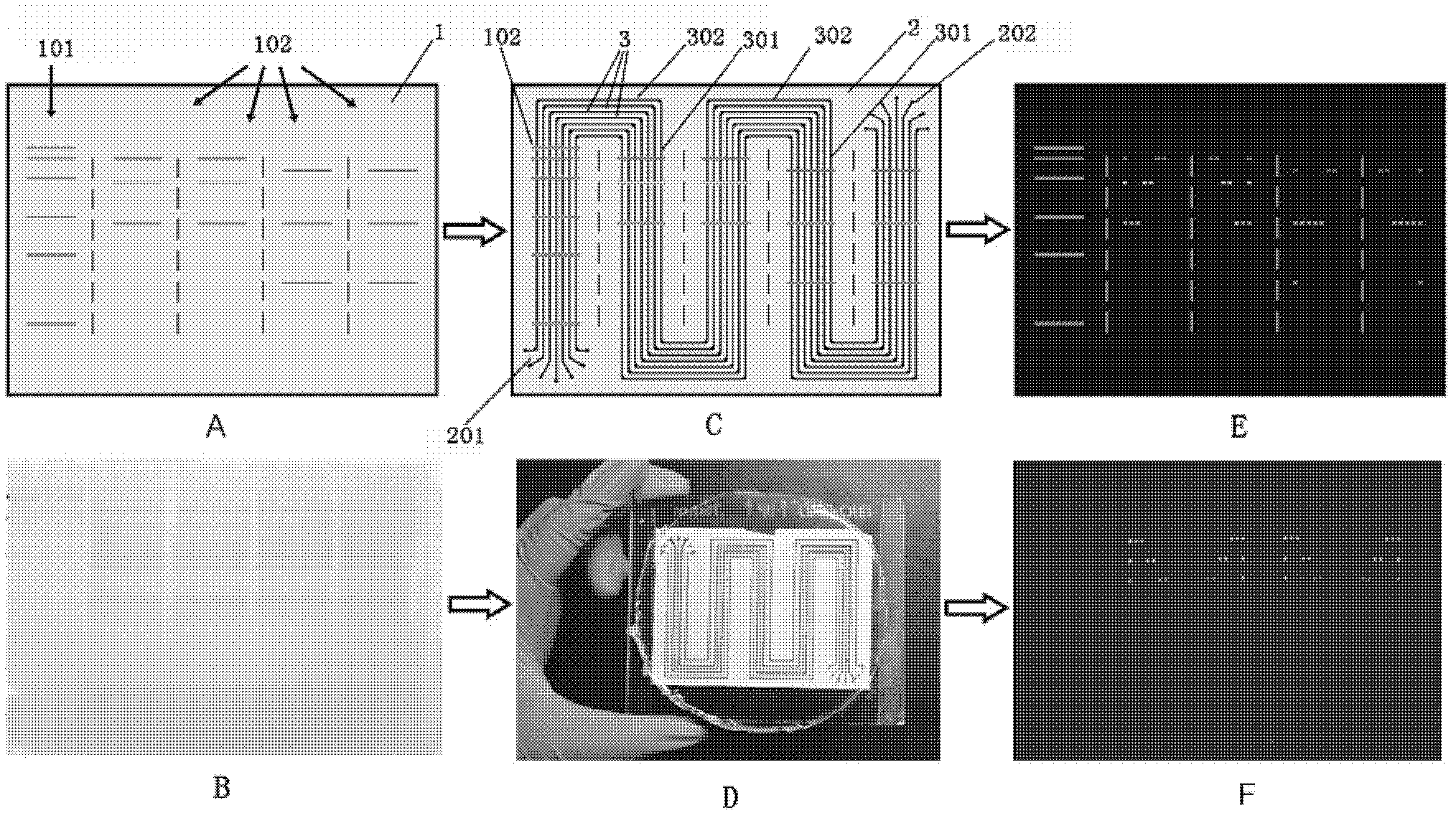
[0045] This example illustrates the preparation method of the multiphase microfluidic immunoblotting chip of the present invention
[0046] 1. Preparation of microfluidic chip template
[0047] The main process of preparation is photolithography, which is to make a photoresist silicon wafer template that is completely consistent with the pattern on the designed mask by using the characteristic that the photoresist can change its properties under ultraviolet irradiation. The specific preparation method can be found in Y Xia, G. Whitesides, Annual Review of Materials Science 1998, 28, 15.
[0048] 2. Preparation of sheet with multiple microfluidic grooves
[0049] The preparation method is soft etching technology, and the preparation material is polydimethylsiloxane (PDMS, poly-dimethylsiloxane), which is a transparent and viscous liquid in a normal state. After being mixed with a curing agent (184 silicone elastomer curing agent , purchased from Dow Corning Corporation of the...
Embodiment 2
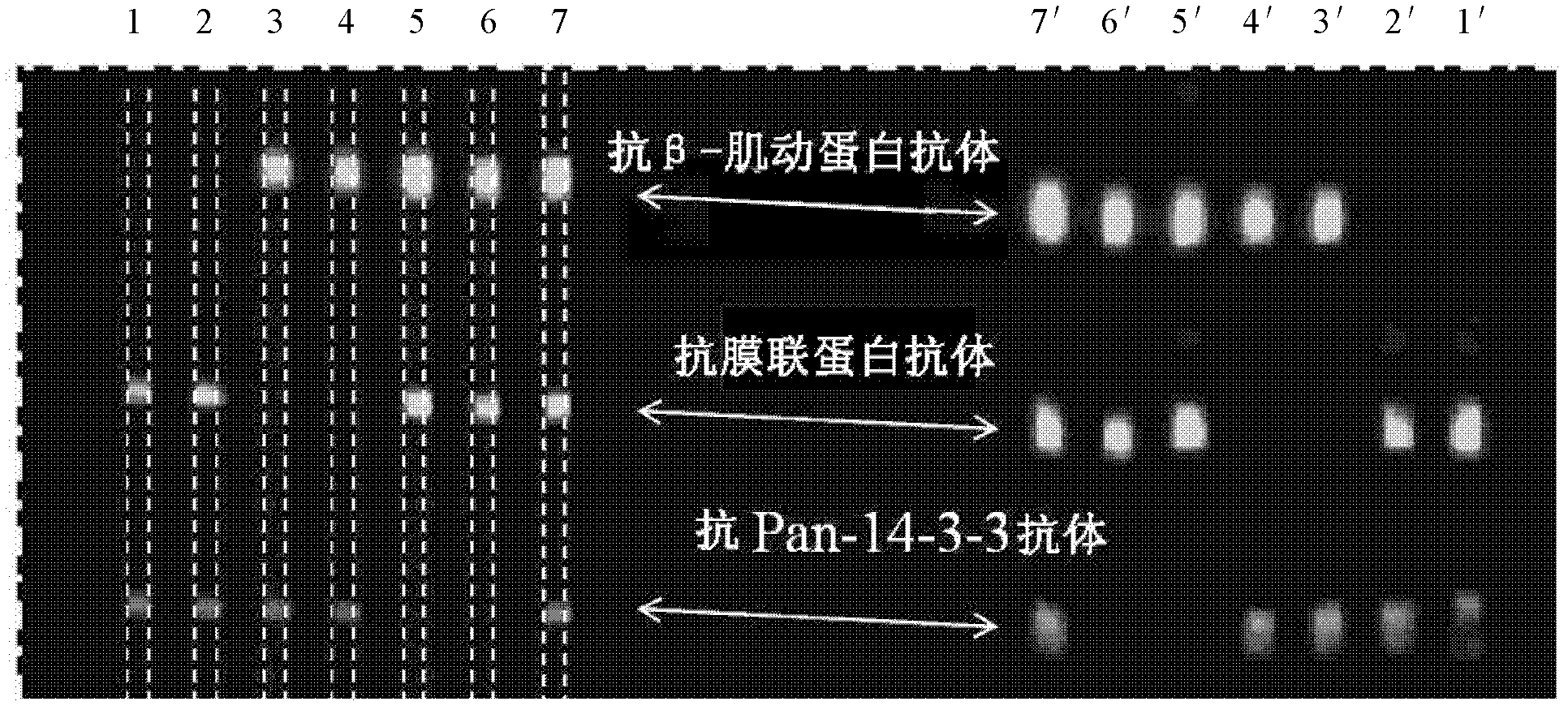
[0081] This example is used to illustrate the multiphase detection capability of the multiphase microfluidic immunoblotting chip of the present invention. The implementation steps are as follows:
[0082] As described in Example 1, load 80 μg of lysed whole protein of NIH 3T3 cells into two swimming lanes, perform electrophoresis according to the preferred time, transfer to membrane, and then dry the membrane loaded with protein, use PDMS monomer and The curing agent was prepared in a mass ratio of 20:1, and baked at 80° C. for 25 minutes to prepare a PDMS chip, and the film and the chip were bonded together by light pressure. From left to right, different antibodies are passed into the channels, specifically, 1 and 2: the mixture of anti-annexin antibody and anti-Pan-14-3-3 antibody; 3 and 4: the passage Mixture of anti-actin antibody (anti-actin antibody) and anti-Pan-14-3-3 antibody; 5 and 6: pass through anti-actin antibody (anti-actin antibody) and anti-membrane Protein...
Embodiment 3
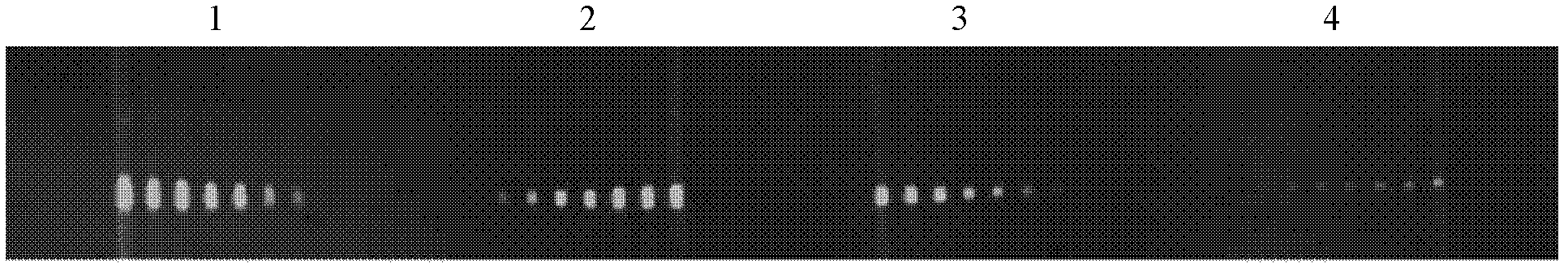
[0084] This example is used to illustrate the ability of the multiphase microfluidic immunoblotting chip of the present invention for semi-quantitative detection.
[0085] As described in Example 1, put 10 μg, 5 μg, 2.5 μg, and 1.25 μg of the lysed whole protein of NIH 3T3 cells in the four lanes, perform electrophoresis according to the preferred time, transfer to the membrane, and then transfer the protein-loaded membrane to Drying, using the mass ratio of PDMS monomer and curing agent at 20:1, drying at 80°C for 25 minutes to prepare a PDMS chip, and combining the film and the chip by light pressure. The anti-annexin antibody from Santa Cruz Company was used at an initial concentration of 1:20, and then the antibody solutions were serially diluted to 1:40, 1:80, 1:160, 1:320, 1:640, and 1:1280. These seven concentrations of antibodies were passed into seven microfluidic channels respectively, and the concentration of the secondary antibody was 1:2000. The dot matrix diagra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Depth | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com