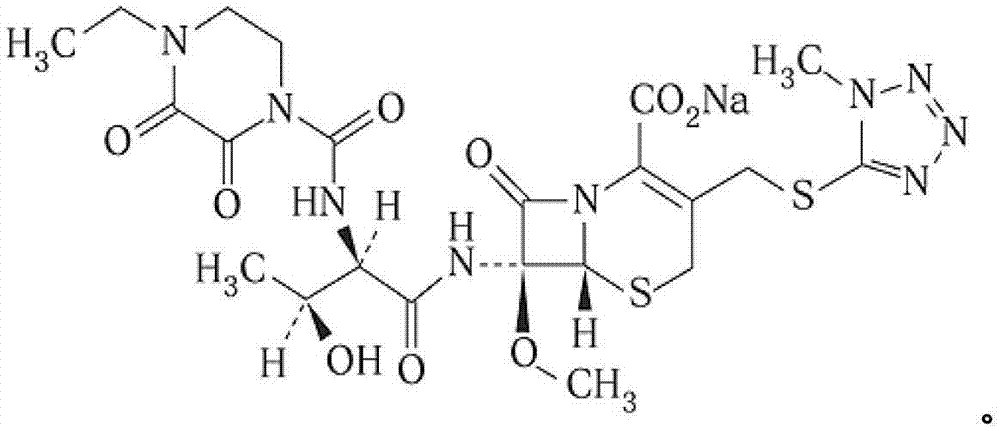
Cefbuperazone sodium composition powder for injection and preparation method thereof
A technology of cefbuperazone sodium and its composition, which is applied in the field of cefbuperazone sodium composition powder for injection and its preparation, can solve the problems of difficulty in obtaining uniform particle shape, poor stability of cefbuperazone sodium, and generation of impurities. Achieve the effect of low requirements on equipment and preparation conditions, stable compatibility and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
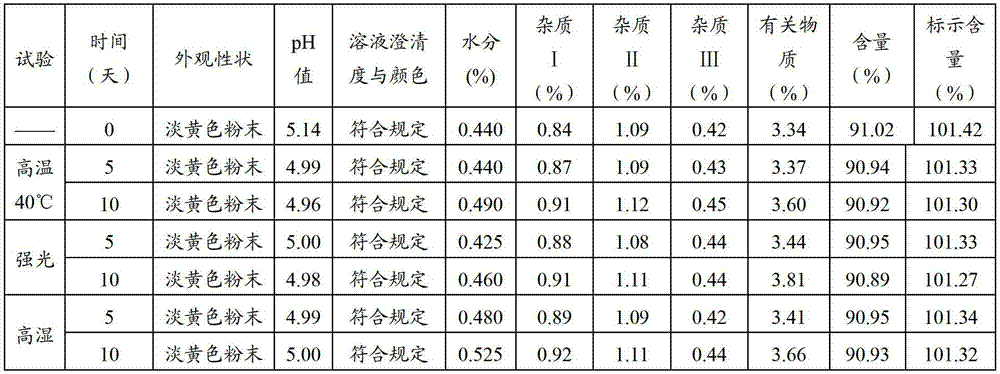
[0028] Screening tests were carried out on the ratio of cefbuperazone sodium and sodium benzoate, and when the dosage ratio was 1:0, 1:0.05, and 1:0.1, they were placed under the condition of relative humidity of 92.5% for 10 days to investigate the moisture; Place it at 40°C for 10 days, and investigate the content change. The results are shown in Table 1 below.
[0029] Table 1 Cefbuperazone Sodium and Sodium Benzoate Proportion Screening Test
[0030] Dosage ratio
[0031] As can be seen from the table 1 results, when not adding sodium benzoate, the hygroscopicity of cefbuperazone sodium is serious, and the degradation increases, and when the ratio of cefbuperazone sodium and sodium benzoate is at 1:0.05, the absorption rate of cefbuperazone sodium can be greatly reduced. Hygroscopicity, improve the stability of the active drug cefbuperazone sodium in cefbuperazone sodium, and ensure that it will not be degraded after being placed for a long time. At a ratio of 1...
Embodiment 2
[0032] Embodiment 2: the cefbuperazone sodium composition powder for injection of the present invention
[0033] Cefbuperazone Sodium 2.6kg
[0034] Sodium Benzoate 0.13kg
[0035] Water for injection 5.2L
[0036] Preparation:
[0037] Step 1: Bottle washing: Take the qualified vials, put them neatly in the stainless steel turntable, and transfer to the bottle washing station. Rinse with purified water and water for injection in turn, blow off residual moisture with compressed air, and enter a 350°C tunnel oven for drying, sterilization, and cooling.
[0038] Step 2: Wash the stopper: Take the chlorinated butyl rubber stopper that has passed the inspection, remove the inner and outer packaging, pour it into the cleaning tank, rinse it with purified water for 20 minutes, then rinse it with water for injection for 20 minutes, and dry it in a drying oven at 121 ± Sterilize at 5°C for 40 minutes, then dry for 20 minutes.
[0039] Step 3: Sterilize the aluminum cap: Send the ...
Embodiment 3
[0044] Embodiment 3: the cefbuperazone sodium composition powder for injection particle diameter and content determination test of the present invention
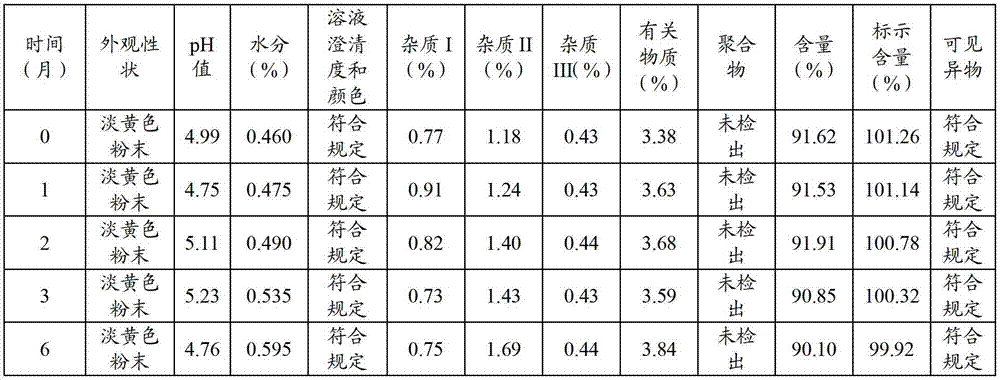
[0045] According to the method of Example 2, three batches of cefbuperazone sodium composition powder for injection were continuously produced, and the obtained product was subjected to particle size determination and content determination, and the measurement results are shown in Table 2.
[0046] Table 2 Cefbuperazone Sodium Composition Powder for Injection Particle Size and Content Determination Test
[0047] the batch
[0048] As can be seen from the results in Table 2, the cefbuperazone sodium composition powder injection particles of the present invention are uniform, and the cefbuperazone sodium content is high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com