Rapid disintegration tabella and chill-pressing method thereof
A disintegrating tablet and disintegrating agent technology, which is applied in the field of pharmaceutical preparations and preparations, can solve problems such as inability to reflect disintegration, and achieve the effects of avoiding poor mold release performance, low solid content, and simple tableting process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
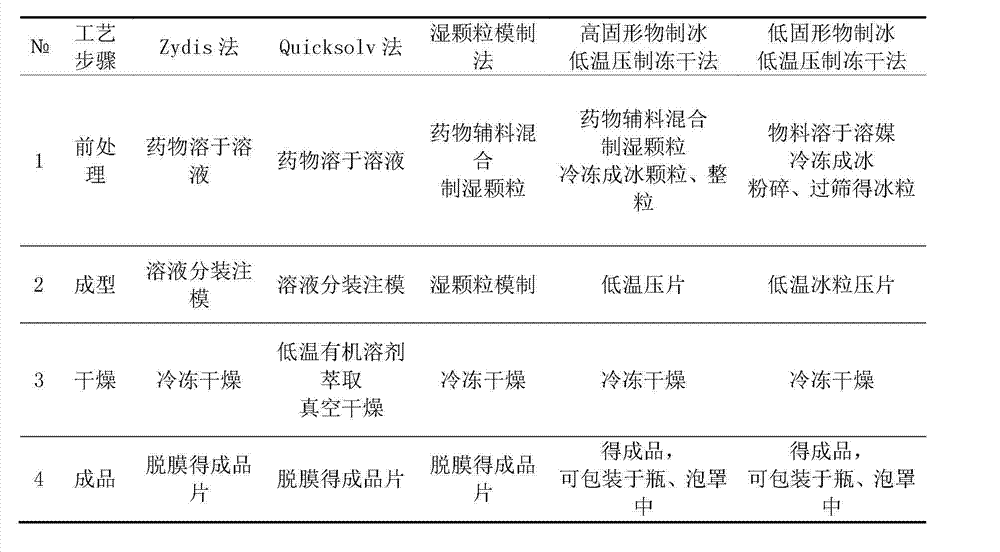
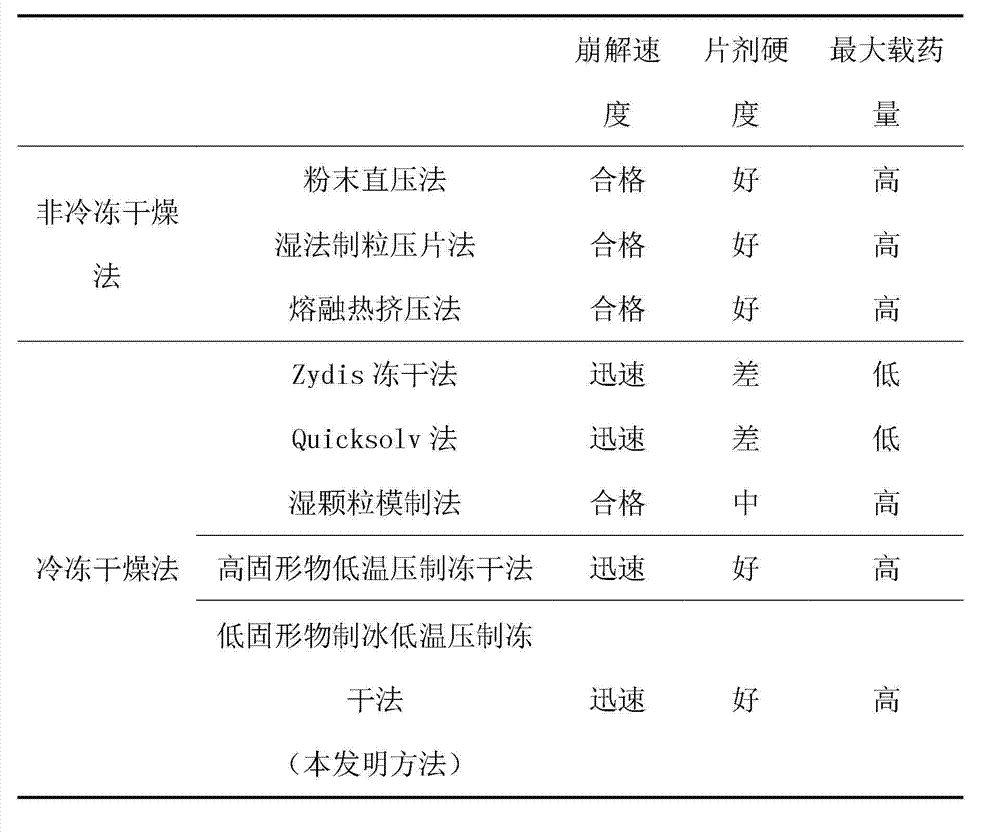
Image
Examples
Embodiment 1
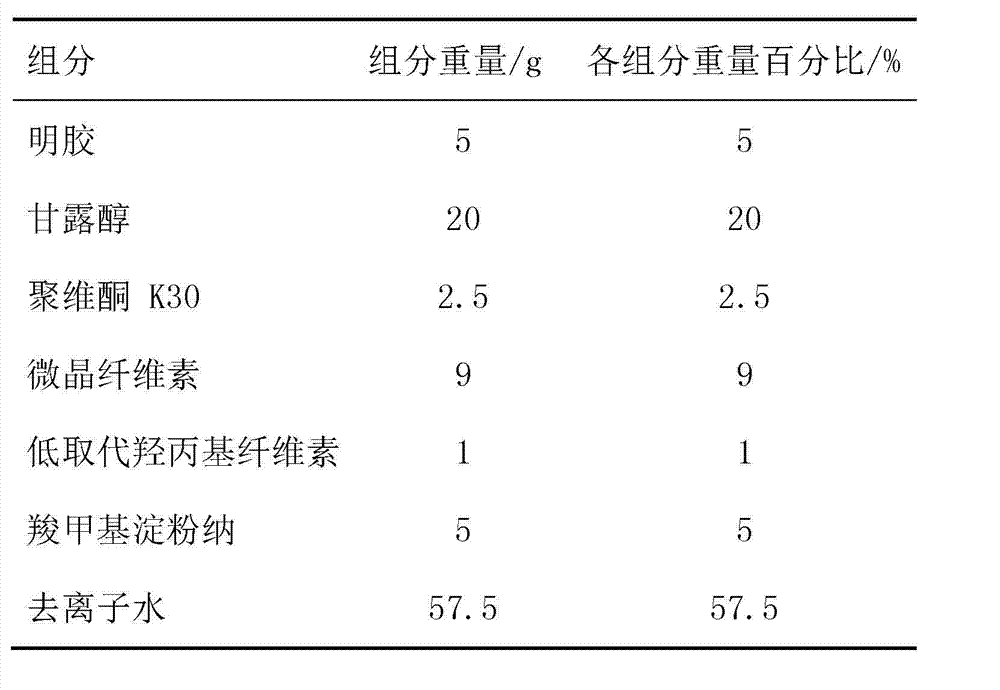
[0038] Compressed tablet core prescription:
[0039]
[0040] Preparation steps:
[0041] 1) Weigh gelatin, mannitol, and PVP K30 according to the prescription; then add the prescribed amount of deionized water; stir and sonicate to obtain a clear solution;
[0042] 2) Put the clarified solution into a freezer to freeze to a solid state;
[0043] 3) Use a pulverizer to crush the solid solution to obtain ice particles, and the ice particles pass through a 50-mesh sieve;
[0044] 4) Weigh the pre-frozen microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and carboxymethyl starch sodium (through a 100-mesh sieve) according to the prescription and mix them evenly, and then mix them evenly with ice particles;
[0045] 5) Press the ice pellets into 500 pieces with a tablet machine;
[0046] 6) The resulting tablet is dried in a freeze dryer to obtain an orally disintegrating tablet.
[0047] The specific freeze-drying parameters and process are as follows: s...
Embodiment 2
[0053] Compressed tablet core prescription:
[0054]
[0055] Preparation steps:
[0056] 1) Weigh gelatin, mannitol, and PVP K30 according to the prescription; then add the prescribed amount of deionized water; stir and sonicate to obtain a clear solution;
[0057] 2) Put the clarified solution into a freezer to freeze to a solid state;
[0058] 3) Use a pulverizer to crush the solid solution to obtain ice particles, and the ice particles pass through a 70-mesh sieve;
[0059] 4) Press the ice pellets into 500 pieces with a tablet machine;
[0060] 5) The resulting tablet is dried in a freeze dryer to obtain an orally disintegrating tablet.
[0061] The freeze-drying process, freeze-drying parameters, disintegration time limit and friability detection are as described in Example 1.
[0062] After testing, the disintegration time limit of the obtained rapidly disintegrating tablets was 35.6 s, and the friability was 0.9%.
Embodiment 3
[0064] Compressed tablet core prescription:
[0065]
[0066] Preparation steps:
[0067] 1) Weigh gelatin and mannitol according to the prescription; then add the prescribed amount of deionized water; stir and sonicate to obtain a clear solution;
[0068] 2) Put the clarified solution into a freezer to freeze to a solid state;
[0069] 3) Use a pulverizer to crush the solid solution to obtain ice particles, and the ice particles pass through a 60-mesh sieve;
[0070] 4) Weigh the pre-frozen povidone K30 (through a 100-mesh sieve) according to the prescription, and then mix it with ice particles evenly;
[0071] 5) Press the ice pellets into 500 pieces with a tablet machine;
[0072] 6) The resulting tablet is dried in a freeze dryer to obtain an orally disintegrating tablet.
[0073] The freeze-drying process, freeze-drying parameters, disintegration time limit and friability detection are as described in Example 1.
[0074] After testing, the disintegration time lim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com