New application of cardiac glycoside compound
A compound and cardiac glycoside technology, which is applied in the field of cardiac glycoside compounds to achieve the effects of good extract quality, simple and easy separation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A, the horn melon ( Calotropis gigantea ) stem 25.4 kg natural air-dried and pulverized, according to the mass / volume ratio (kg / L) of croissant melon sample: ethanol aqueous solution of about 1:2, 50 L of ethanol aqueous solution with a volume fraction of 95% and 25.4 kg crocus melon sample Fully mix, seal, and extract at room temperature for 3 times, each time for 7 days, filter and combine the extracts, and concentrate under reduced pressure until there is no alcohol smell to obtain the ethanol extract;
[0026] B. At room temperature, disperse the ethanol extract in water to make a suspension, according to the volume ratio of ethanol extract: petroleum ether = 1:3, 1:2, 1:1, repeat the ethanol extract with petroleum ether After extracting 3 times, concentrating under reduced pressure and recovering petroleum ether, petroleum ether extract (254.1 g) was obtained;
[0027] C. Filter the water liquid after petroleum ether extraction, separate the filtrate by D-101 macr...
Embodiment 2
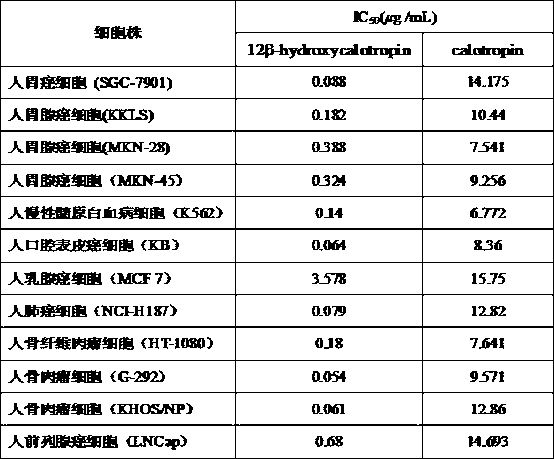
[0032] After column chromatography, Fr.4-1 (3.2 g) in step E in Example 1 was eluted with chloroform:methanol=25:1 volume ratio eluent, and the same components were collected by TLC thin layer chromatography , to obtain 7 parts Fr.4-1-1~Fr.4-1-7; Fr.4-1-4 (869.5 mg) was subjected to silica gel column chromatography, followed by chloroform:methanol=40:1, chloroform : The eluent of acetone=6:2 volume ratio is eluted, finally obtains cardiac glycoside compound calotropin (109.9 mg). The study found that the structural difference between this compound and the target compound of the present invention is that there is no hydroxyl group on the 12-position carbon, while the 12-position carbon of the target compound of the present invention is substituted by a hydroxyl group. In further in vitro antitumor activity tests, the anti-IC 50 The values are significantly different (see Table 2).
Embodiment 3
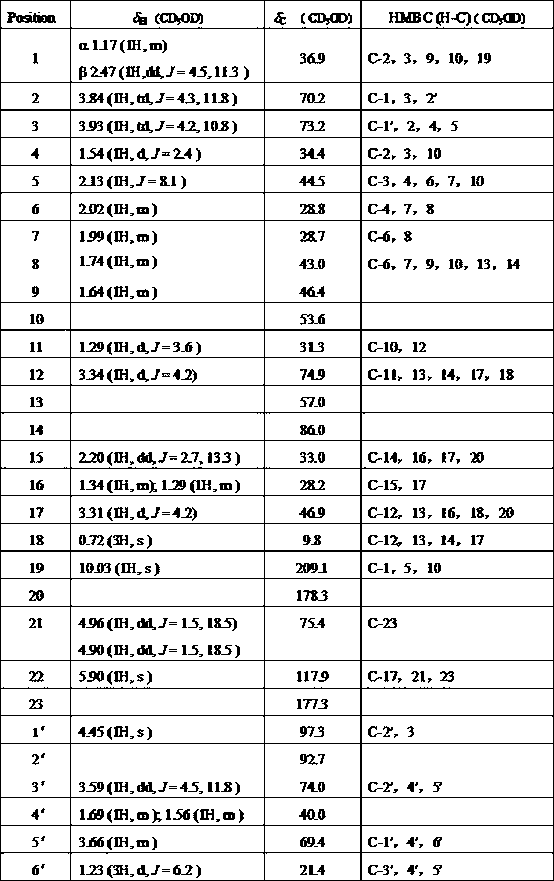
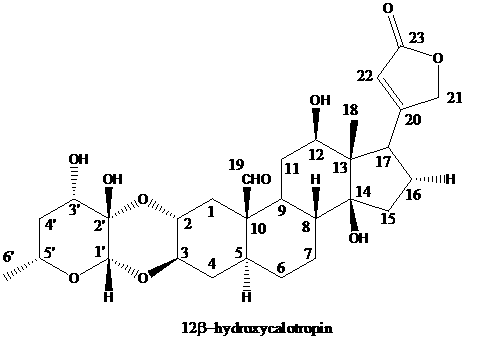
[0034] Structural identification of cardiac glycoside compound 12β-hydroxycalotropin:
[0035]The structure of the cardiac glycoside compound 12β-hydroxycalotropin in Example 1 was identified by spectroscopic techniques, including ultraviolet, infrared, nuclear magnetic resonance and high-resolution mass spectrometry. The detection of the cardiac glycoside compound 12β-hydroxycalotropin for the first time in the stem of the horn squash by using 2D-NMR technology 13 C-NMR, 1 The H-NMR data were assigned (see Table 1 below).
[0036] The following are cardiac glycoside compounds 12β-hydroxycalotropin The physical and chemical constants:
[0037] 12β-hydroxycalotropin: C 29 h 40 o 10 , white amorphous powder (methanol), 10% sulfuric acid ethanol solution is brown. m.p. 214-216 °C; [α] D +6.4° (c = 0.045, MeOH); HR-ESI-MS: m / z [M+Na] + 583.2318 ( calcd. For C 29 h 40 o 10 Cl, 583.2310); IRλ max (cm -1 ): 3433 cm -1 , 2957 cm -1 , 2923 cm -1 , 2852 cm -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com