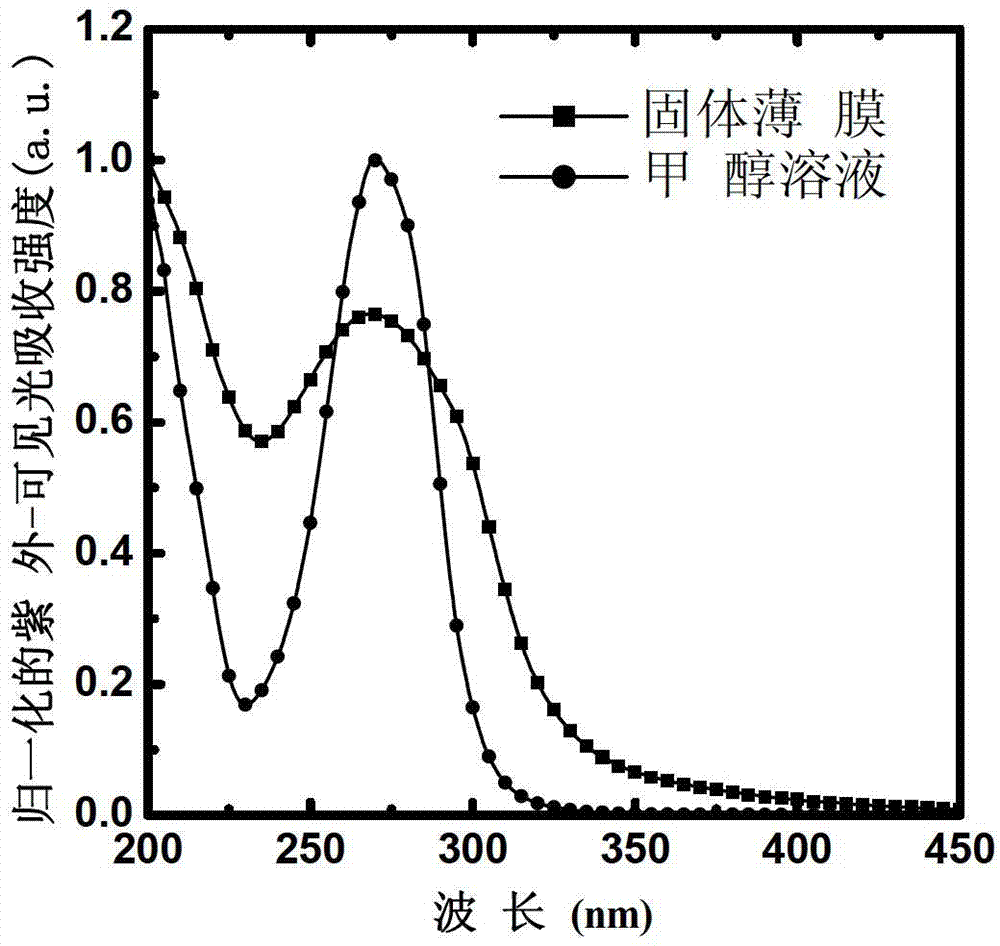
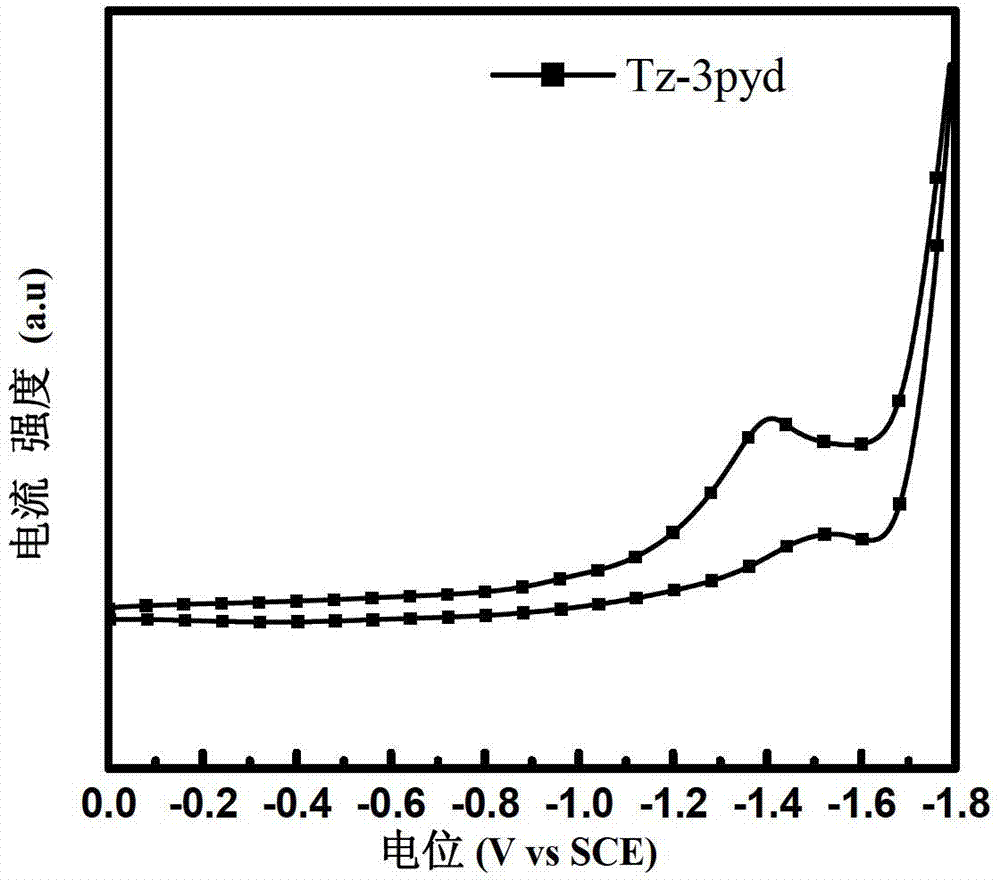
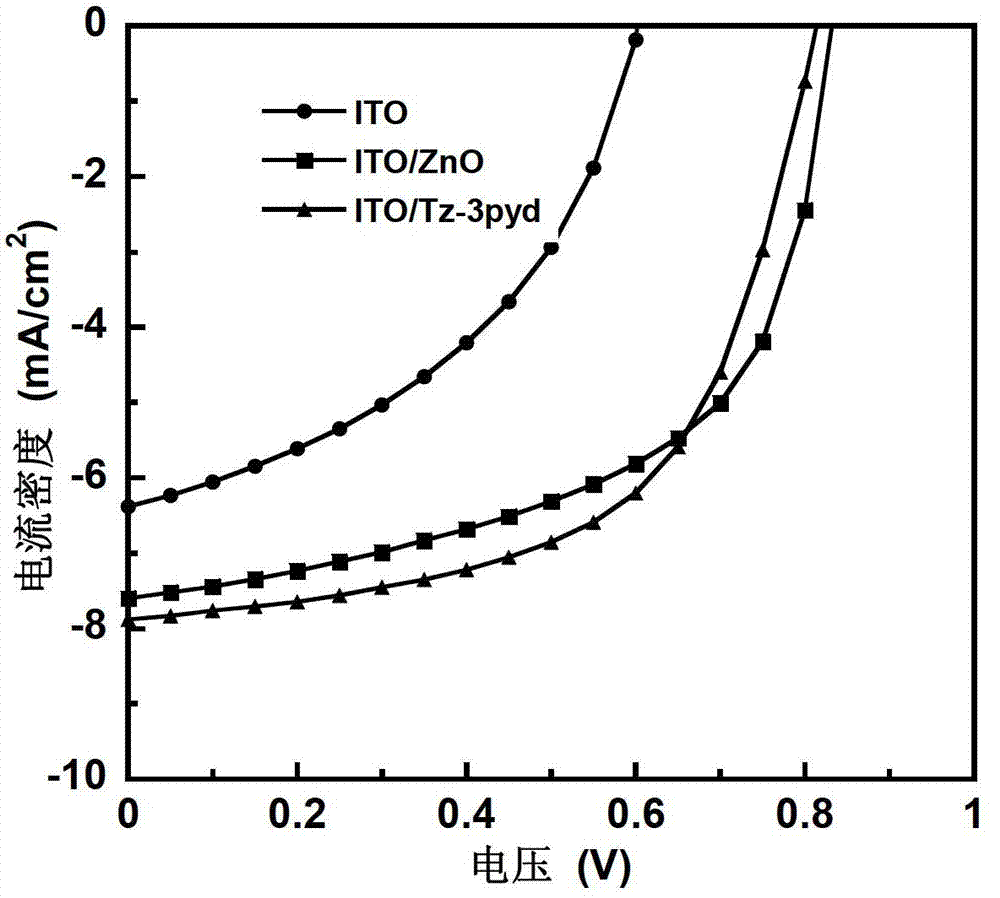
Water-soluble small molecule pyridinium photoelectric material and preparation method and application thereof
A molecular pyridinium salt, photoelectric material technology, applied in photovoltaic power generation, chemical instruments and methods, circuits, etc., can solve the uncertainty of polymer molecular weight, purity and structure, it is difficult to ensure the performance stability of different batches of materials, high Cost of vacuum evaporation process and other issues, to avoid the uncertainty of purity and molecular weight, good carrier injection/extraction characteristics, and low electron injection/extraction energy barriers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of the water-soluble small molecule pyridinium salt photoelectric material of the present embodiment comprises the following steps:
[0043] Step 1: the synthesis of p-bromomethylbenzonitrile, as follows:
[0044]
[0045] Under nitrogen protection, add p-tolunitrile (5.05g, 43mmol) and 50ml of carbon tetrachloride to a 150ml reaction vessel and stir, then add N-bromosuccinimide (7.69g, 43mmol) in Stir at room temperature for 10 minutes. Finally, the catalyst benzoyl peroxide (0.63g.2.0mmol) was added and heated to 80°C for 5 hours in the dark. After the temperature dropped to 60°C, the resulting filtrate was filtered under reduced pressure to obtain a light yellow solid. Recrystallized from a mixed solvent of absolute ethanol: ethyl acetate = 3:1 to obtain 4.67 g of white crystals, with a yield of 55.2%. The nuclear magnetic resonance result of the intermediate product that step one obtains is as follows: 1 H NMR (300MHz, CDCl 3 ), (ppm):...
Embodiment 2
[0056] The preparation method of the water-soluble small molecule pyridinium salt photoelectric material of the present embodiment comprises the following steps:
[0057] Under the protection of nitrogen, add pyridine and acetonitrile into the reaction vessel, heat and stir at 65°C, then add the chloromethylated aromatic heterocyclic compound, wherein the molar ratio of the chloromethylated aromatic heterocyclic compound to pyridine is 1:16, reacted at 70°C for 11 hours, cooled to room temperature, and removed the solvent under reduced pressure. After the crude product was dissolved in methanol, it was precipitated and filtered in ethyl acetate several times. The water-soluble small molecule pyridinium salt material is obtained by vacuum drying.
[0058] The chloromethylated aromatic heterocyclic compound has the following structure:
[0059]
[0060] The water-soluble small molecule pyridinium salt optoelectronic material prepared in this example has the following structu...
Embodiment 3
[0069] The preparation method of the water-soluble small molecule pyridinium salt photoelectric material of the present embodiment comprises the following steps:
[0070] Under the protection of nitrogen, add pyridine and acetonitrile into the reaction vessel, heat to 60°C and stir, then add bromomethylated aromatic heterocyclic compound, wherein the molar ratio of bromomethylated aromatic heterocyclic compound to pyridine is 1:16, reacted at 90°C for 10 hours, cooled to room temperature, and removed the solvent under reduced pressure. After the crude product was dissolved in methanol, it was precipitated and filtered in ethyl acetate several times. The water-soluble small molecule pyridinium salt material is obtained by vacuum drying.
[0071] The bromomethylated aromatic heterocyclic compound has the following structure:
[0072]
[0073] The water-soluble small molecule pyridinium salt photoelectric material prepared in this embodiment has the following chemical structu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com