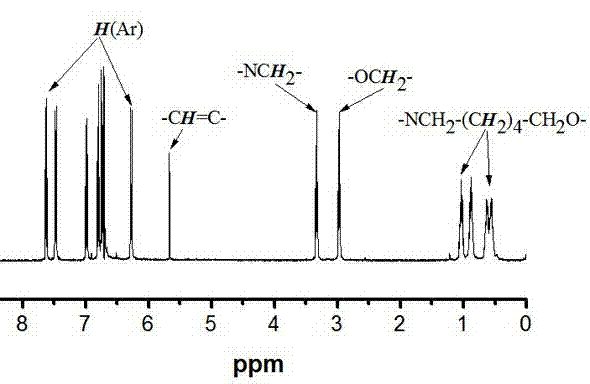
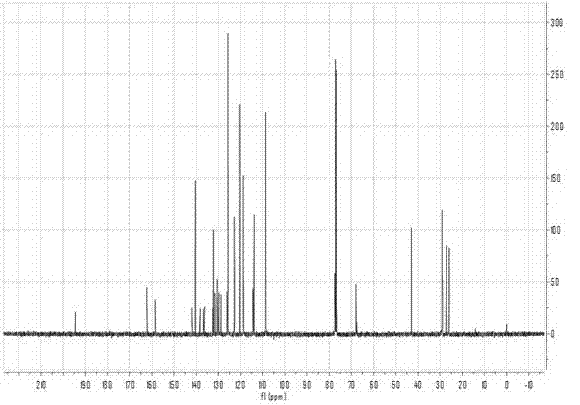
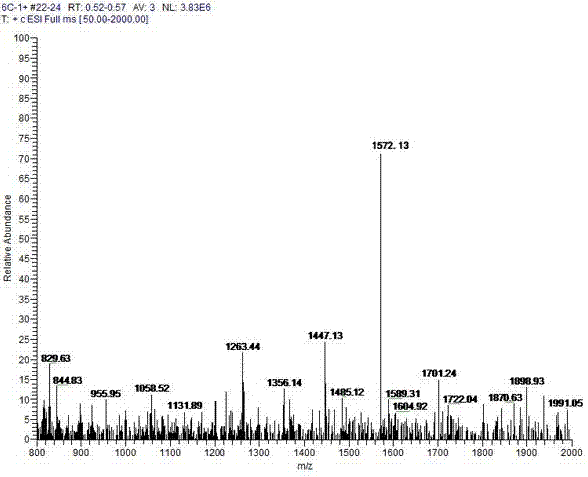
Aggregation-induced emission material with long alkyl chain toluylene carbazole structure, synthesizing method and application thereof
An aggregation-induced luminescence, stilbene carbazole-based technology, applied in luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problem of fluorescent efficiency and lifespan of luminescent materials with service life, which has not been well solved, and OLED devices. The fluorescence efficiency and service life cannot meet the practical requirements, and the OLED display cannot enter the industrial scale application, etc., to achieve the effects of good solubility, easy purification, and good luminous characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 4,4'-bis(2,2'-bis(4-(6-(9H-carbazolyl)hexyloxy)phenyl)vinyl)biphenyl
[0029] (1) Synthesis of intermediate compound N-6-bromohexyl-9H-carbazole
[0030] In a 100ml three-necked flask equipped with a reflux device and protected by nitrogen, 3g (0.018mol) carbazole, 35ml acetone, 4.3g KOH, and 18ml (0.115mol) 1,6-dibromohexane were sequentially added. Stir at room temperature for 24 h, then pour into water, extract 3 times with dichloromethane, wash the organic phase with water 4 times, wash with anhydrous Mg 2 SO 4 After drying for 3 h, the solvent was distilled under reduced pressure. The remaining solid was separated by column chromatography (SiO 2 ; Eluent: dichloromethane / n-hexane=1:6, v / v), to obtain a white solid product.
[0031]
[0032] (2) Synthetic intermediate bis(4-(6-(9H-carbazolyl)hexyloxy)phenyl)methanone
[0033] In a 100ml three-neck flask equipped with a reflux device and protected by argon, add 1g (3mmol) compound N-6-bromohexyl-...
Embodiment 2
[0043] Synthesis of 4,4'-bis(2,2'-bis(4-(12-(9H-carbazolyl)dodecyloxy)phenyl)vinyl)biphenyl
[0044] (1) Synthesis of intermediate N-12-bromododecyl-9H-carbazole
[0045] In a 100ml three-necked flask equipped with a reflux device and protected by nitrogen, 3g (0.018mol) carbazole, 35ml acetone, 4.3g KOH, and 38g (0.115mol) 1,12-dibromododecane were sequentially added. Stir at room temperature for 24 h, then pour into water, extract 3 times with dichloromethane, wash the organic phase with water 4 times, wash with anhydrous Mg 2 SO 4 After drying for 3h, it was distilled under reduced pressure. The remaining solid was separated by column chromatography (SiO 2 ; Eluent: dichloromethane / n-hexane=1:6, v / v), to obtain a white product.
[0046]
[0047] (2) Synthetic intermediate bis(4-(12-(9H-carbazolyl)dodecyloxy)phenyl)methanone
[0048] In a 100ml three-necked flask equipped with a reflux device and protected by argon, 1.7g (3mmol) N-12-bromododecyl-9H-carbazole, 20ml D...
Embodiment 3
[0054] Synthesis of 4,4'-bis(2,2'-bis(4-(4-(9H-carbazolyl)butoxy)phenyl)vinyl)biphenyl
[0055] (1) Synthesis of intermediate compound N-4-bromobutyl-9H-carbazole
[0056] In a 100ml three-necked flask equipped with a reflux device and protected by nitrogen, add 3g (0.018mol) carbazole, 35ml acetone, 0.02g TBAB, 4.3g KOH, and 25g (0.115mol) 1,4-dibromobutane in sequence. Stir at room temperature for 24 h, then pour into water, extract 3 times with dichloromethane, wash the organic phase with water 4 times, wash with anhydrous Mg 2 SO 4 After drying for 3 h, the solvent was distilled under reduced pressure with a rotary evaporator. The remaining solid was separated by column chromatography (SiO 2 ; Eluent: dichloromethane / n-hexane=1:6, v / v) to obtain a white product.
[0057]
[0058] (2) Synthesis of bis(4-(4-(9H-carbazolyl)butoxy)phenyl)methanone
[0059] In a 100ml three-necked flask equipped with a reflux device and protected by argon, add 0.9g (3mmol) N-4-bromobuty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com