D-dimer quality control product and preparation method thereof
A technology for constitution and inhibitor, which is applied in the field of preparing D-dimer quality control products by using bovine plasma, can solve the problems of unsuitability for commercial production, high price, and difficulty in obtaining, and achieves easy acquisition, low production cost, and storage time. prolonged effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D- 2
[0022] The preparation of embodiment 1D-dimer quality control product
[0023] 1. Bovine plasma: Add 3.8% trisodium citrate anticoagulant solution to the blood collected from the jugular vein of cattle, and the volume ratio of blood to anticoagulant is 9:1. The blood added with anticoagulant was centrifuged for 30 minutes with a relative centrifugal force of 2000g, and the upper layer of plasma was carefully separated and drawn.
[0024] 2. Lyoprotectant: TAPSO1.3%, sodium chloride 2%, sodium azide 1.0g / L, mannitol 10g / L, bovine serum albumin 20g / L, aprotinin 80000KIU / L.
[0025] 3. Preparation of D-dimer quality control:
[0026] (1) Divide the bovine plasma into glass test tubes, add 0.025mol / L calcium chloride solution with 40% plasma volume to each tube, and bathe in water at 37°C for 15 minutes until the plasma coagulates.
[0027] (2) Add plasmin solution to the plasma to make the final concentration 240U / mL, place it in a 37°C water bath for 6 hours, and stir it with ...
Embodiment 2D- 2
[0030] Embodiment 2D-dimer quality control product is compared to reagent change sensitivity
[0031] 1. Accurately reconstitute the D-dimer quality control product prepared in Example 1 and the commercially available D-dimer quality control product from Siemens, Germany.
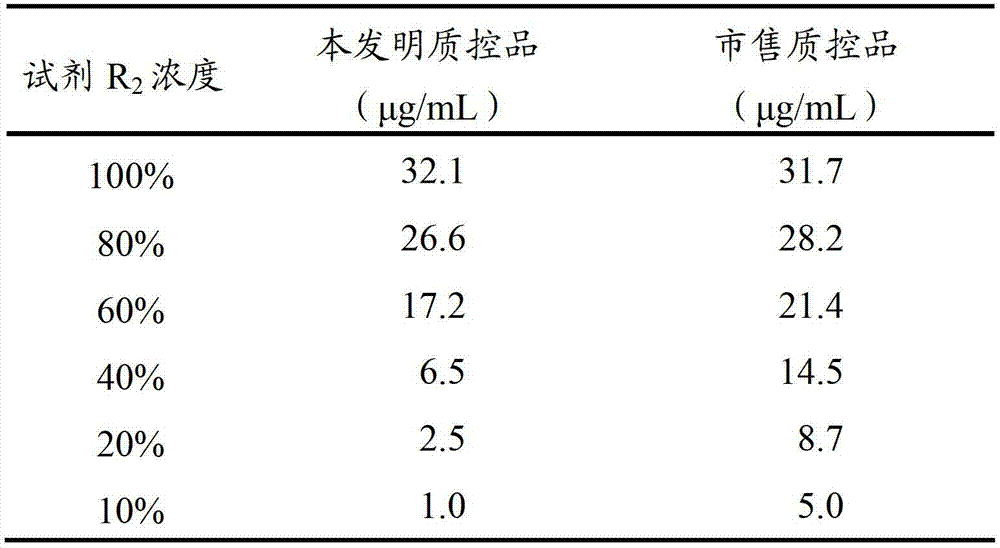
[0032] 2. Use the D-dimer detection kit of Siemens company in Germany to detect D-dimer, and the detection method is latex immunoturbidimetric method. R in the kit 2 The reagent (immune latex reagent) was serially diluted with distilled water to obtain R 2 The concentration is: 100%, 80%, 60%, 40%, 20%, 10% reagent.
[0033] 3. Use serially diluted R 2 Reagents and other reagents in the kit, the D-dimer quality control product prepared in Example 1 and the commercially available D-dimer quality control product from Siemens, Germany were tested, and the test results are shown in Table 1. The result shows: detection reagent R 2 Concentration from 100% to 10%, the D-dimer quality control product of the pr...
Embodiment 3D- 2
[0036] Example 3D - Detection of the stability of the dimer quality control product.
[0037] 1. Open bottle stability test
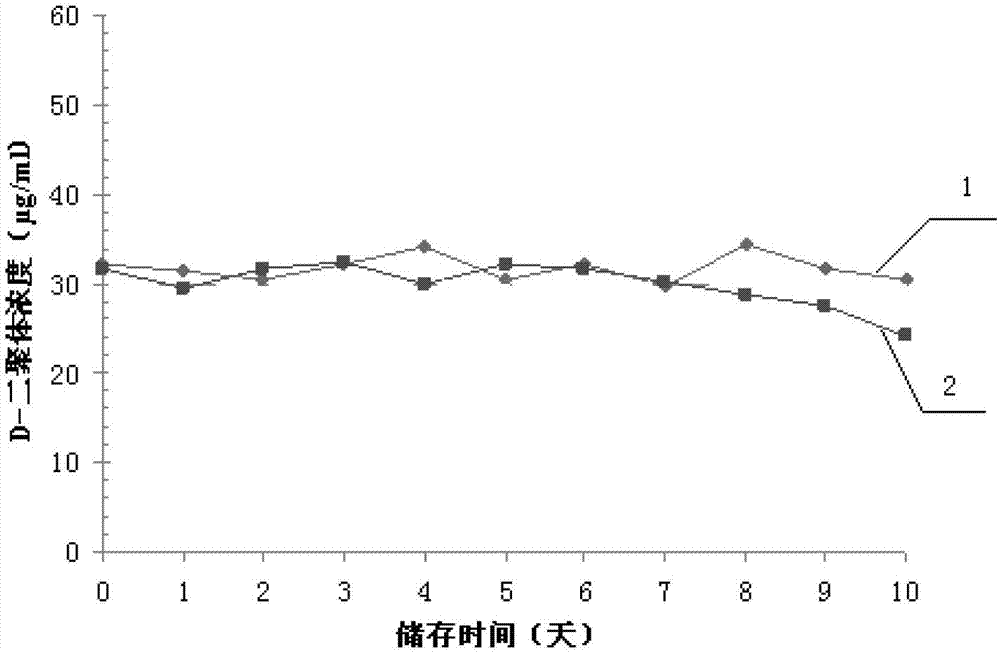
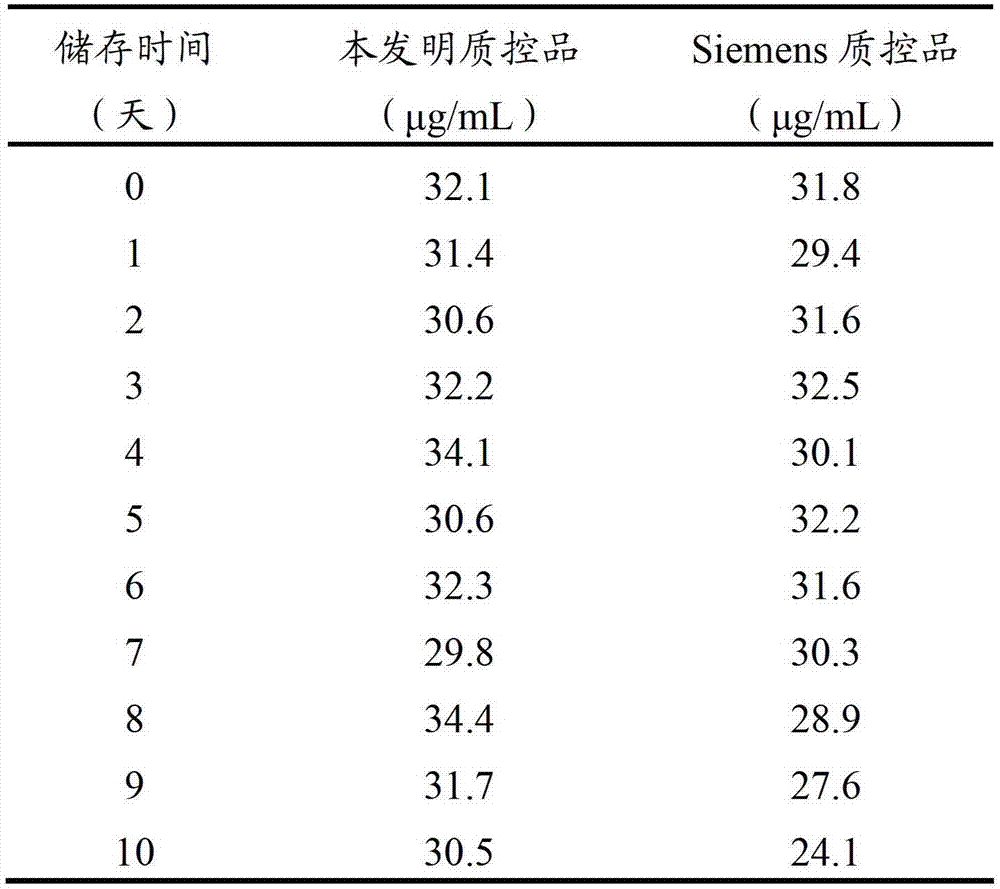
[0038] The D-dimer quality control product prepared in Example 1 and the German Siemens company quality control product were accurately reconstituted respectively, stored at room temperature (15-25°C), and regularly sampled once a day to detect the D-dimer quality control product concentration. The linear regression method was used to analyze the stability of the quality control substance, and the slope b of the linear equation was tested to see whether there was a significant difference from 0. If P0.05, it indicated that the concentration of the analyte was between It is basically stable during the measurement time. The test results are shown in Table 2 and figure 1 shown.
[0039] Table 2 Open bottle stability test results
[0040]
[0041] The regression equation of the quality control product of the present invention is: y=-0.0182x+31.882, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com