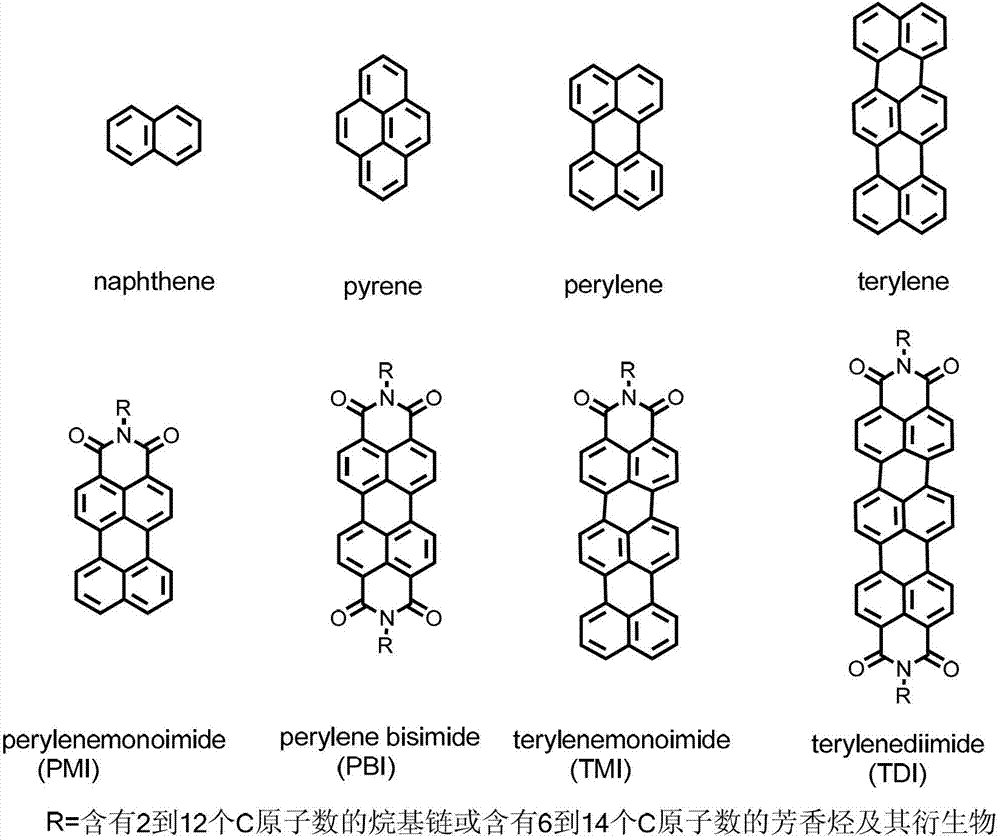
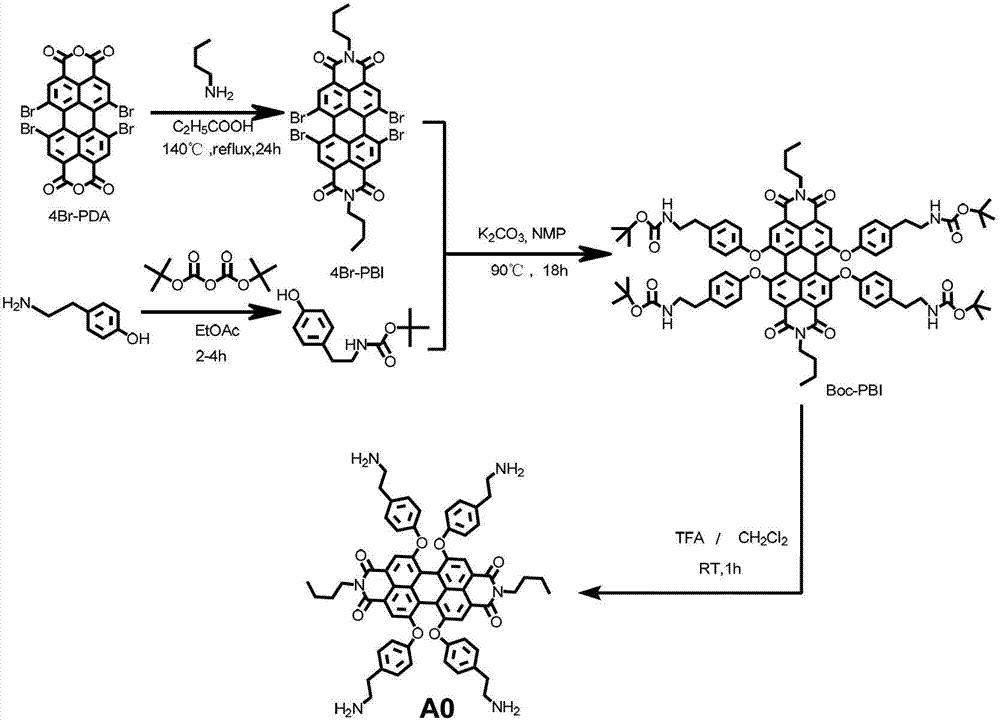
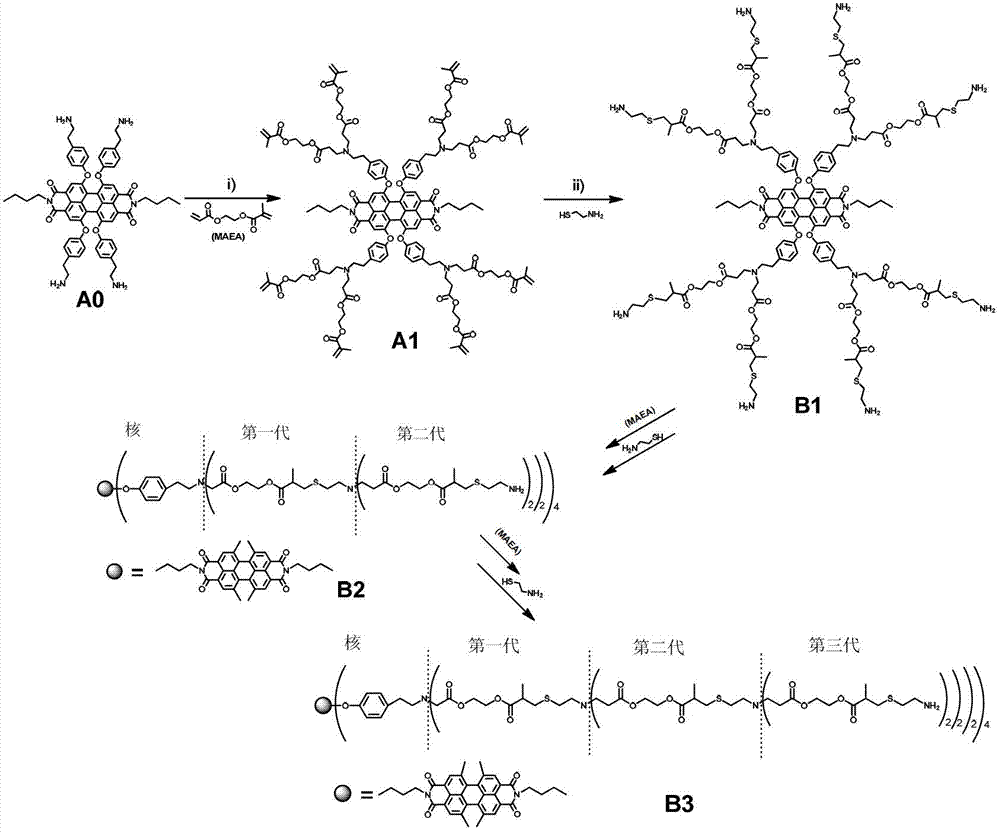
Synthetic method and application of water-soluble fluorescent dendrimers
A synthesis method and macromolecular technology, applied in the field of gene carriers of non-viral systems, can solve the problems of structural defects of dendritic macromolecules, complicated synthesis steps, accompanied by side reactions, etc., and achieve reduction of synthesis and purification steps and low cytotoxicity. , the effect of excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of Fluorescent Dendrimers Using Click Chemistry
[0043] (1) Synthesis of the first-generation fluorescent dendrimers with terminal methacrylates (A1)
[0044] Add 0.16g (0.16mmol) perylene compound A0 carrying four primary amino groups and 0.2g (3.15mmol) MAEA into a 10mL two-port reaction tube, stir overnight at room temperature under a nitrogen atmosphere, then react at 50°C for 48h, etc. After the reaction temperature dropped to room temperature, the reaction liquid was washed with n-hexane 3 times, each time with 30 mL of n-hexane, and vacuum-dried to obtain the red oily product A1 with a yield of 98%.
[0045] 1 H-NMR (400Hz, CDCl 3 ):δppm:8.20(s,4H),7.09(d,8H),6.91(d,8H),6.11(s,8H),5.56(d,8H),4.32(br,32H),4.12(t, 4H),2.87(t,16H),2.69(m,16H),2.51(t,16H),1.9(s,24H),1.59(m,4H),1.36(m,4H),0.90(t,6H ).MS(MALDI-TOF,m / z)Calc.for C 136 h 158 N 6 o 40 ,2516.73;found:2515.8.
[0046] (2) Synthesis of amino-functionalized first-generation fluore...
Embodiment 2
[0059] Except that in step (1), propyl methacryloxyacrylate is used instead of ethyl methacryloxyacrylate to react with the perylene compound A0 carrying four primary amino groups, other operations are the same as in Example 1 , to prepare the first to third generation perylene fluorescent dendrimers.
Embodiment 3
[0061] In addition to using ethyl methacryloxyethoxyethoxy acrylate in step (1) instead of ethyl methacryloxy acrylate to react with the perylene compound A0 carrying four primary amine groups, other operations Same as in Example 1, the first to third generation perylene fluorescent dendrimers were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com