Preparation method of fluorocarbon/hydrocarbon mixed type cation surface active monomer
A technology of surface active monomer and hydrocarbon mixed type, which is applied in the preparation of fluorine-containing cationic surface active monomers and the preparation of quaternary ammonium salt type surface active monomers, can solve harsh reaction conditions, difficult product purification, and reaction yields. low rate and other problems, to achieve the effect of simple processing, convenient product collection, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, preparation fluorine-containing surface-active monomer
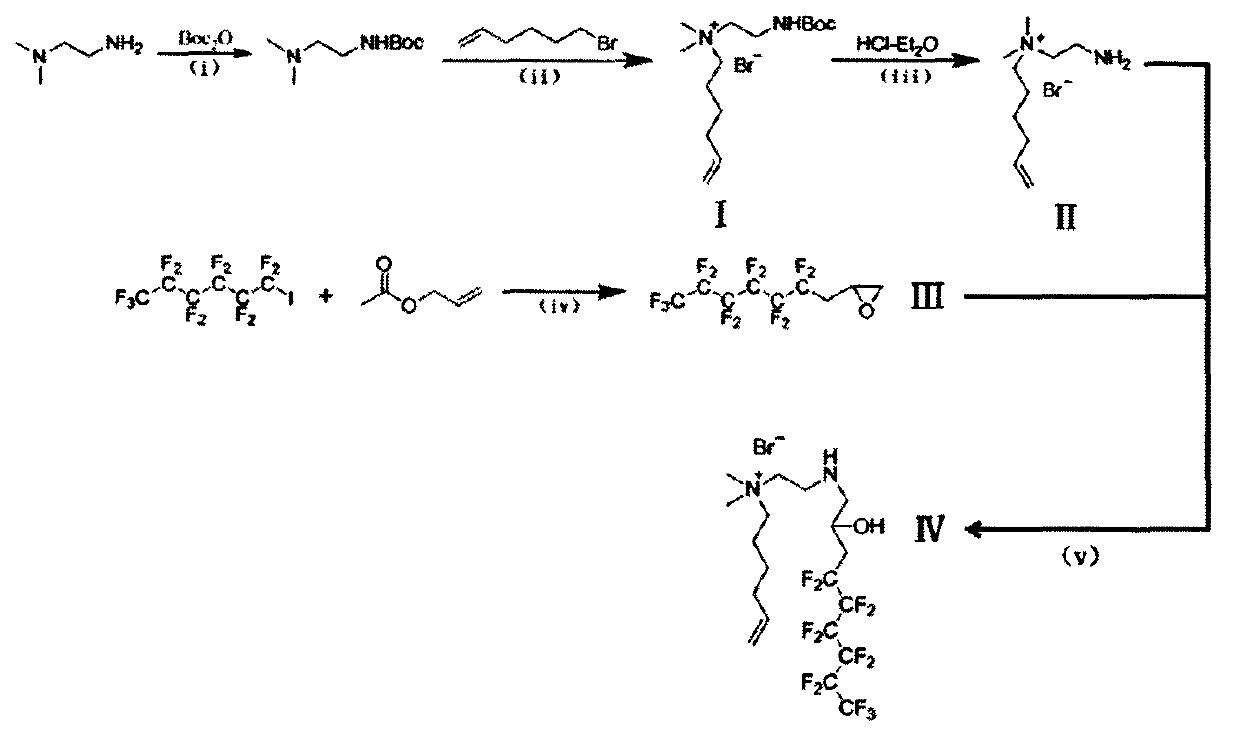
[0045] (1) Preparation of compound I
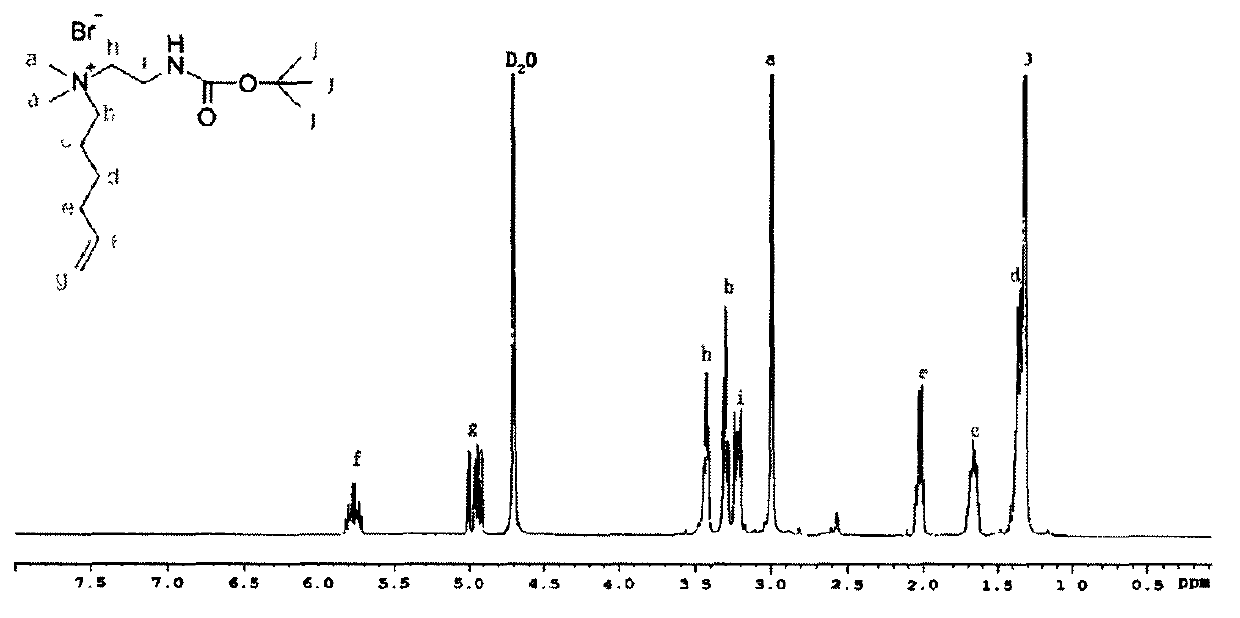
[0046] Weigh 0.88g (10mmol) N, N-dimethylethylenediamine and dissolve it in 15mlDMF, transfer it to the 2 Drying tube), nitrogen tube, and constant pressure dropping funnel in a 50ml three-neck round bottom flask, then put it into a constant temperature water bath at 25°C, add the rotor, start stirring, and pass through nitrogen to remove oxygen for 30min. Weigh 2.62g (12mmol) of Boc anhydride and dissolve in 10ml of DMF, then transfer to a 25ml constant pressure dropping funnel. Open the cock, drop the Boc anhydride dissolved in DMF into the three-neck flask drop by drop, after 0.5h, remove the constant pressure dropping funnel. After the mixture was reacted for 1 h, 2.45 g (15 mmol) of 6-bromo-1-hexene was dissolved in 5 ml of DMF and transferred to a 10 ml constant pressure dropping funnel. Open the cock, drop by drop into the above-mentioned three-necked ...
Embodiment 2
[0053] Embodiment 2, preparation fluorine-containing surface-active monomer
[0054] (1) Preparation of compound I
[0055] As described in Example 1, the difference is that the quality of Boc anhydride is changed to 2.40g (11mmol), the quality of 6-bromo-1-hexene is changed to 1.79g (11mmol), and the quality of gained compound I is 2.85g , the yield was 81.2%.
[0056] (2) Preparation of compound II
[0057] As described in Example 1, the difference is that the volume of the saturated HCl-diethyl ether solution was changed to 7ml, the mass of compound II obtained was 2.17g, and the yield was 94.2%.
[0058] (3) Preparation of compound III
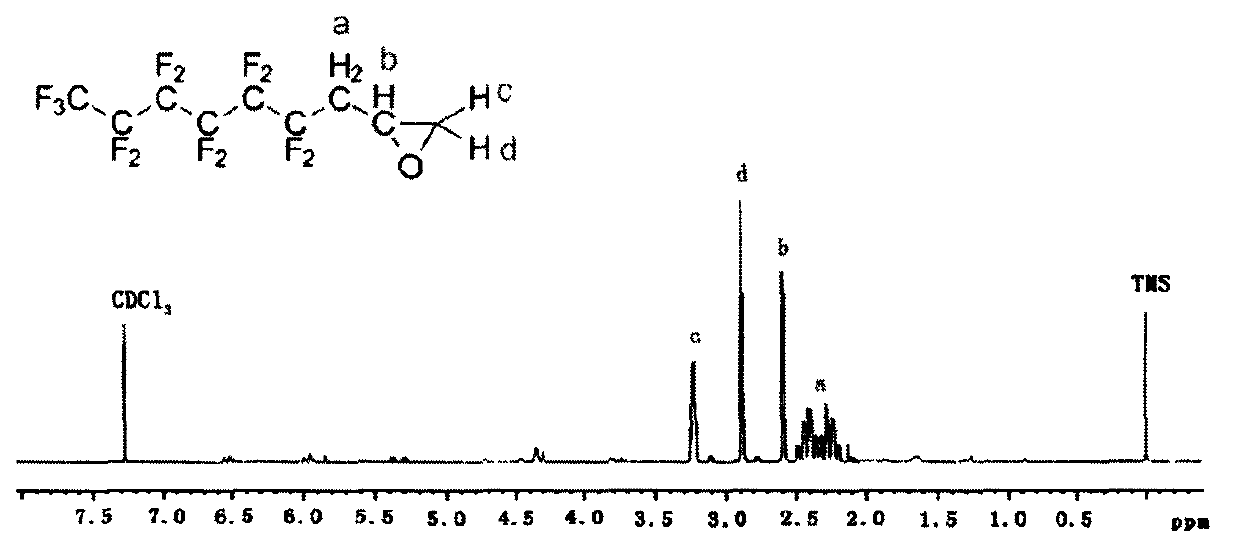
[0059] As described in Example 1, the difference was that the mass of BPO was changed to 0.242 g (1.0 mmol), and the mass of compound III obtained was 7.07 g, with a yield of 62.7%.
[0060] (4) Preparation of Compound IV
[0061] As described in Example 1, except that the mass of compound III was changed to 6.02 g (16 mmol), the mass...
Embodiment 3
[0062] Embodiment 3, preparation fluorine-containing surface-active monomer
[0063] (1) Preparation of compound I
[0064] As described in Example 1, the difference is that the quality of Boc anhydride is changed to 2.40g (11mmol), the quality of 6-bromo-1-hexene is changed to 1.63g (10mmol), and the quality of gained compound I is 2.88g , the yield was 82.3%.
[0065] (2) Preparation of compound II
[0066] As described in Example 1, the difference is that the volume of the saturated HCl-ether solution was changed to 10 ml, and the obtained compound II had a mass of 2.23 g and a yield of 96.8%.
[0067] (3) Preparation of compound III
[0068] As described in Example 1, except that the mass of BPO was changed to 0.169g (0.7mmol), the mass of compound III obtained was 7.42g, and the yield was 65.8%.
[0069] (4) Preparation of Compound IV
[0070] As described in Example 1, the difference was that the mass of compound III was changed to 4.52 g (12 mmol), and the mass of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com