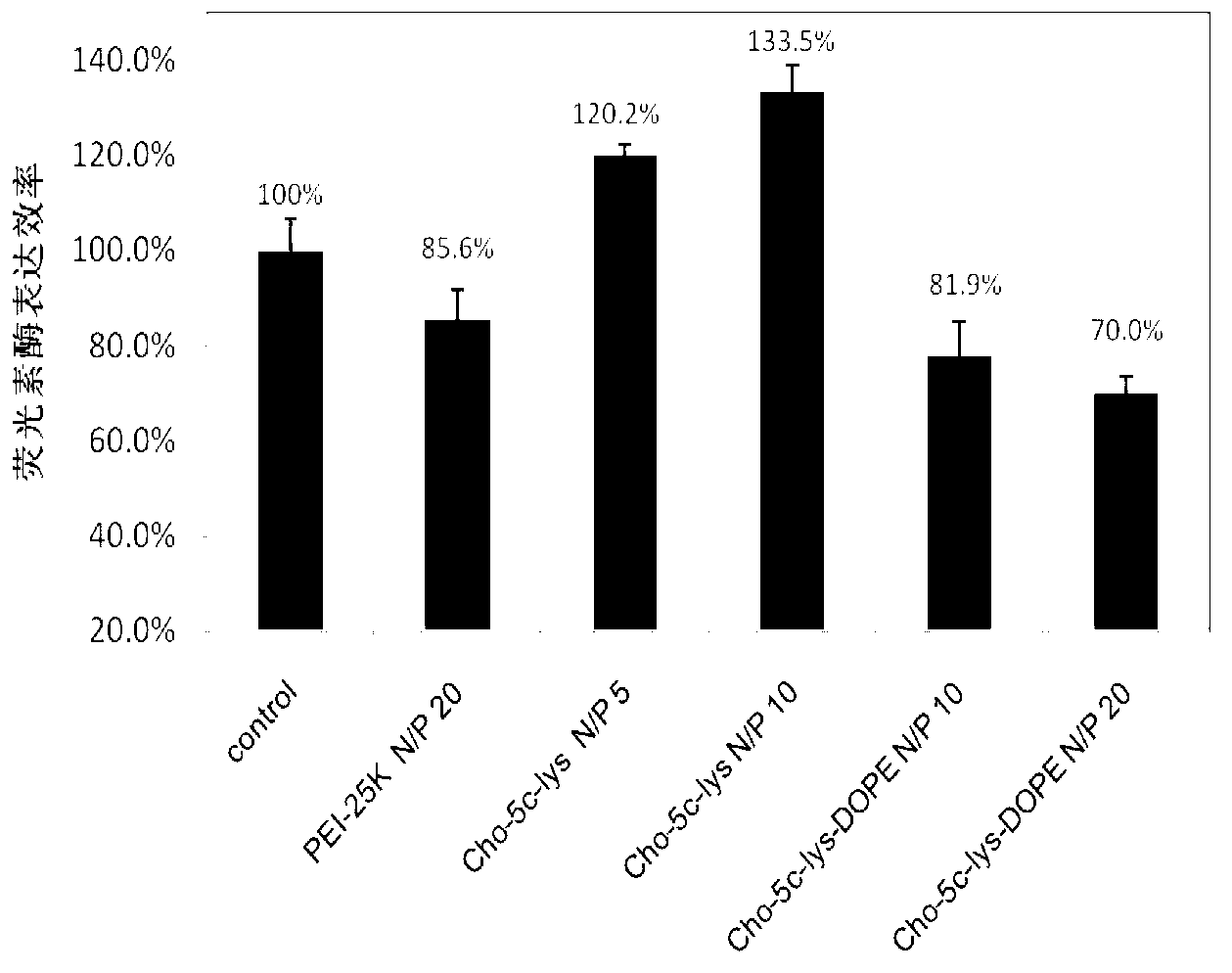Organic functional molecule containing natural cholesterol and lysine lipid cations, lipidosome thereof, as well as preparation method and application for lipidosome
A technology of functional molecules and cholesterol, which is applied in the preparation of lipid-like cationic organic functional molecules and their liposomes, and the application field of small-molecule interfering RNA transfection reagents, achieving simple and efficient preparation methods, high siRNA transfection efficiency, and ease of use. The effect of low-cost large-scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Lipid molecule Cho-5C-Lys
[0033] The first step: pentylene glycol (12.2g, 0.1mmol) is dissolved in 30ml dry dichloromethane, above-mentioned solution is slowly added dropwise to the cholesterol chloroformate (4.5 g, 0.01mol) solution, the reaction was stirred at 30°C for 12h, and then the organic solvent was distilled off. After column chromatography (eluent: ethyl acetate / petroleum ether=1 / 2v / v), the cholesterol monosubstituted pentanediol carbonate intermediate was prepared. The synthesis yield was 80%.
[0034] 1 H NMR (CDCl 3 , 300MHz): 5.32(s, 1H, C=CH, cholesterol), 4.54(t, 2H, J=6.3Hz, CH 2 OCO), 4.51(m, 1H, OCOCH), 4.36(t, 2H, J=6.3Hz, CH 2 OCO), 3.79(t, 2H, J=6.3Hz, CH 2 O), 2.30-0.95 (m, 45H, cholesterol)
[0035] ESI-MS: [M + ]=531.5m / z
[0036] The second step: Dissolve BOC-protected natural lysine (3.5g, 0.01mol) in 50ml dry chloroform, and slowly add it dropwise to the previous step dissolved in organic solvent under the catalysis of...
Embodiment 2
[0040]
[0041] Lipid molecule Cho-4C-Lys
[0042] The first step: butanediol (9.6g, 0.1mmol) was dissolved in 50ml dry dioxane, and the above solution was slowly added dropwise to cholesterol p-toluenesulfonate dissolved in 50ml dry dioxane containing 20ml pyridine (5.4g, 0.01mol) solution, stirred and reacted at 40°C for 18h, and then distilled off the organic solvent. After column chromatography (eluent: ethyl acetate / petroleum ether=1 / 2v / v), the cholesterol monosubstituted butanediol carbonate intermediate was obtained. The synthetic yield is 71%.
[0043] 1 H NMR (CDCl 3 ,300MHz): 5.32(s, 1H, C=CH, cholesterol), 3.50(t, 2H, J=6.3Hz, CH 2 OH), 3.37(t, 2H, J=5.1Hz, CHOCH 2 ), 2.30-0.95 (m, 45H, cholesterol)
[0044] ESI-MS: [M + ]=503.4m / z
[0045] Step 2: Dissolve BOC-protected natural lysine (3.5g, 0.01mol) in 50ml of dry tetrahydrofuran, and slowly add it dropwise to the previous step dissolved in 20ml of dry tetrahydrofuran under the catalysis of 0.5g of 4-di...
Embodiment 3
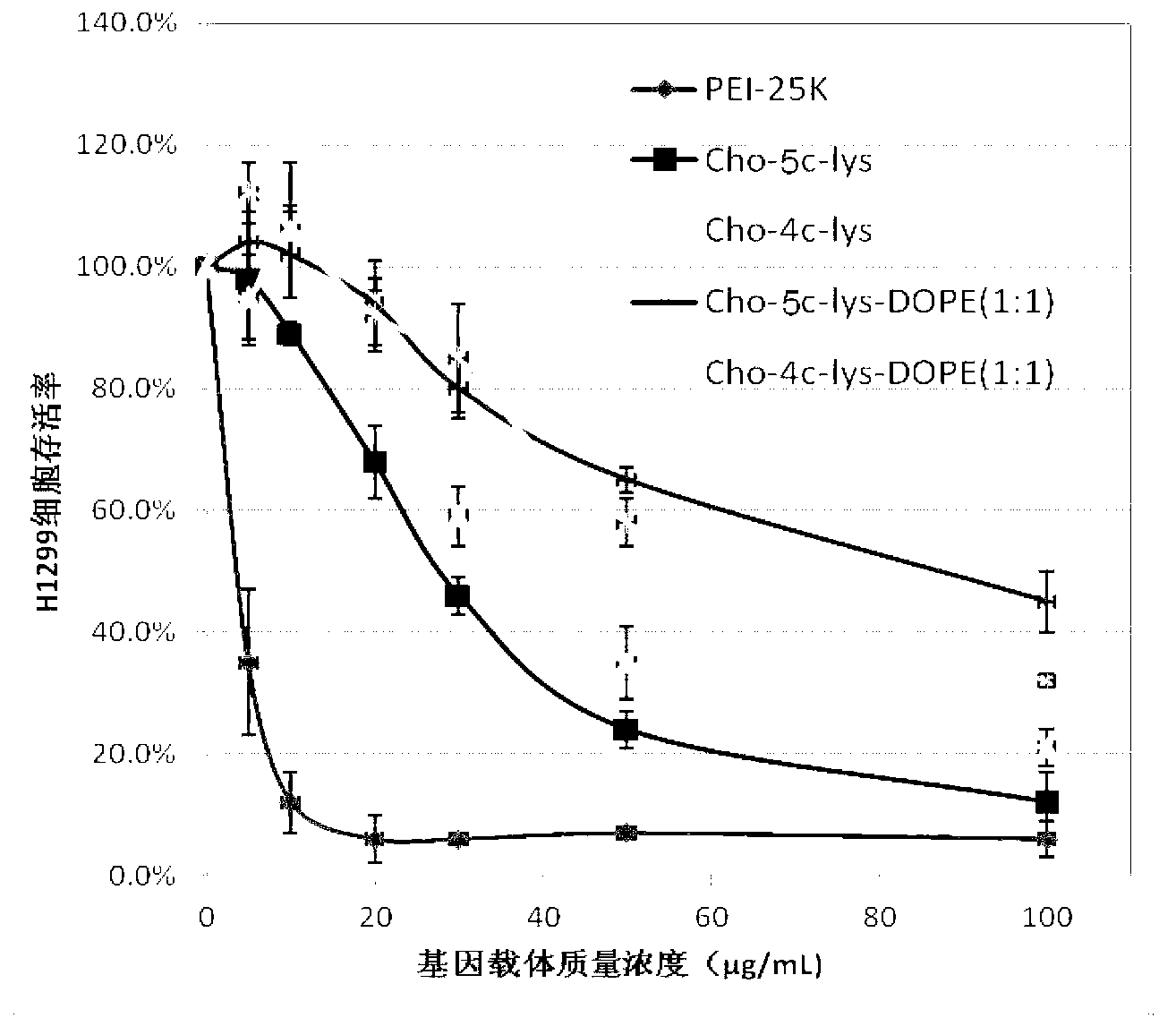
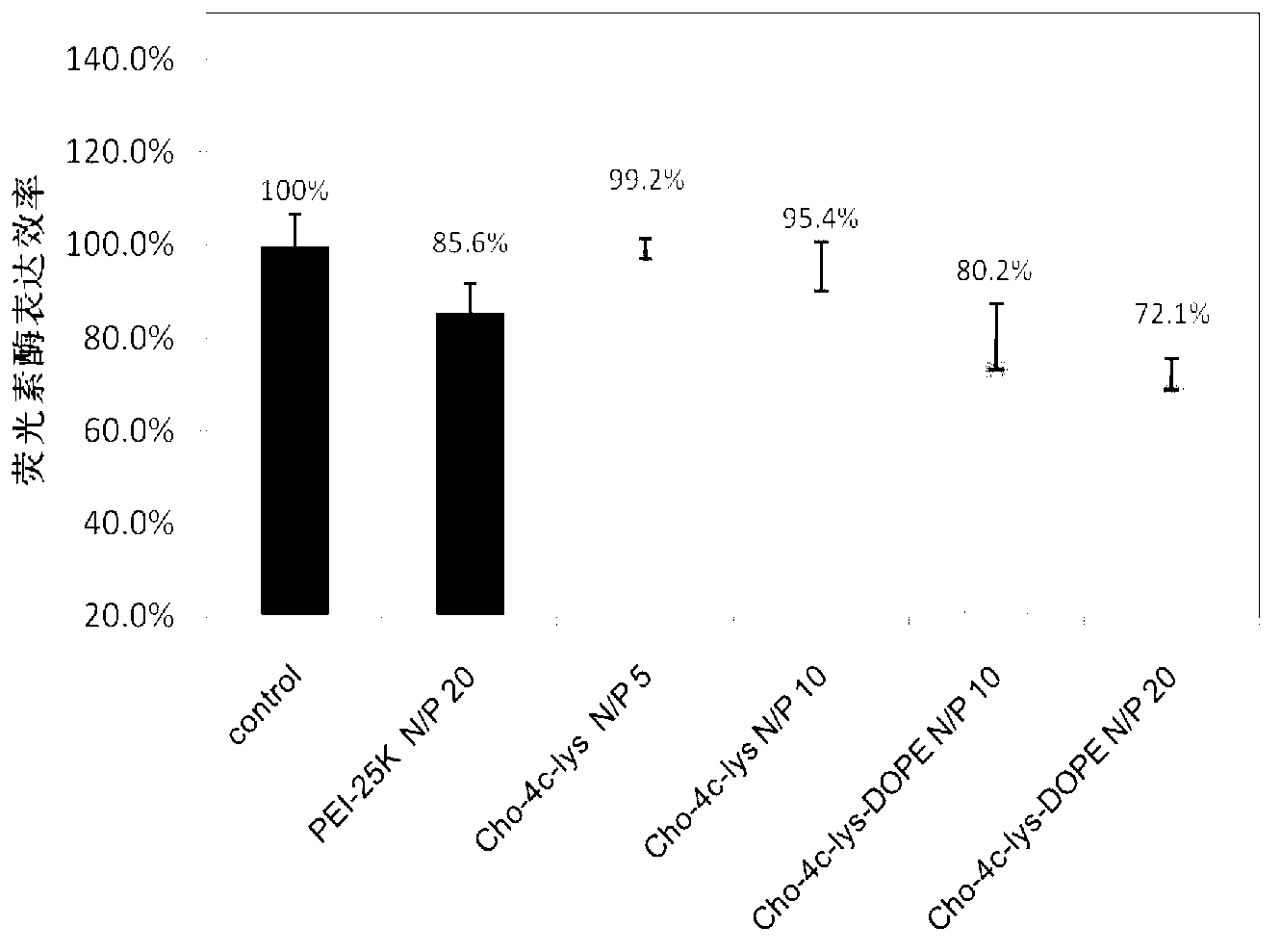
[0049] In a 250mL round bottom flask, weigh 1mmoL of the lipid-like cationic organic functional molecule Cho-5C-Lys synthesized above containing natural cholesterol and lysine, and then add dioleoylphosphatidylethanolamine with a molar ratio of 1:1 (DOPE) mixed with it. Then fully dissolve the mixed lipid in 100mL chromatographic grade chloroform solution, and then remove the chloroform solvent in a rotary evaporator at 40°C. After a uniform lipid film is formed at the bottom of the bottle, add 100mL deionized water, ultrasonic water After 0.5h, liposome Cho-5C-Lys-DOPE containing natural cholesterol and lysine was obtained.
[0050] Particle size of liposome Cho-5C-Lys-DOPE Surface Potential of Liposome Cho-5C-Lys-DOPE 108.6±12.3nm 23.1±2.5mV
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com