Preparation method of ion imprinted polymer film
A technology of polymer film and ion imprinting, applied in separation methods, chemical instruments and methods, membrane technology, etc., can solve problems such as unsuccessful technical solutions, and achieve the effects of convenient control, simple and fast operation, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0037] (1) 0.3423 g pyrrole monomer is dissolved in the aqueous solution of 200 ml and is mixed with the pyrrole aqueous solution A of 0.025 mol / L;
[0038] (2) 3.7462 g potassium chloride is dissolved in the aqueous solution of 100 ml and is mixed with the potassium chloride aqueous solution B of 0.5 mol / L;
[0039] (3) 4.8627 g concentrated hydrochloric acid is dissolved in the aqueous solution of 100 ml and is mixed with the hydrochloric acid solution C of 0.5 mol / L;
[0040] (4) 0.6064 g nickel chloride is dissolved in the aqueous solution of 100 ml and is mixed with the nickel chloride solution D of 0.025 mol / L;
[0041] (5) 0.8273 g potassium ferricyanide is dissolved in the aqueous solution of 100 ml and is mixed with the potassium ferricyanide solution E of 0.025 mol / L;
[0042] (6) Take 10 ml of solution A, solution B, solution C, solution D and solution E respectively into the reaction vessel and stir evenly;
Embodiment approach 2
[0048] (1) 0.3423 g pyrrole monomer is dissolved in the aqueous solution of 200 ml and is mixed with the pyrrole aqueous solution A of 0.025 mol / L;
[0049] (2) 3.7462 g potassium chloride is dissolved in the aqueous solution of 100 ml and is mixed with the potassium chloride aqueous solution B of 0.5 mol / L;
[0050] (3) 4.8627 g concentrated hydrochloric acid is dissolved in the aqueous solution of 100 ml and is mixed with the hydrochloric acid solution C of 0.5 mol / L;
[0051] (4) 0.6064 g nickel chloride is dissolved in the aqueous solution of 100 ml and is mixed with the nickel chloride solution D of 0.025 mol / L;
[0052] (5) 0.8273 g potassium ferricyanide is dissolved in the aqueous solution of 100 ml and is mixed with the potassium ferricyanide solution E of 0.025 mol / L;
[0053] (6) Take 10 ml of solution A, solution B, solution C, solution D and solution E respectively into the reaction vessel and stir evenly;
Embodiment approach 3
[0058] (1) 0.3423 g pyrrole monomer is dissolved in the aqueous solution of 200 ml and is mixed with the pyrrole aqueous solution A of 0.025 mol / L;
[0059] (2) 5.055 g potassium nitrate is dissolved in the aqueous solution of 100 ml and is mixed with the potassium nitrate aqueous solution B of 0.5 mol / L;
[0060] (3) Dissolve 4.8469 g of concentrated nitric acid in 100 ml of aqueous solution and prepare 0.5 mol / L nitric acid solution C;
[0061] (4) 0.9576 g yttrium nitrate is dissolved in the aqueous solution of 100 ml and is mixed with 0.025 mol / L yttrium nitrate solution D;
[0062] (5) 0.8273 g potassium ferricyanide is dissolved in the aqueous solution of 100 ml and is mixed with the potassium ferricyanide solution E of 0.025 mol / L;
[0063] (6) Take 10 ml of solution A, solution B, solution C, solution D and solution E respectively into the reaction vessel and stir evenly;
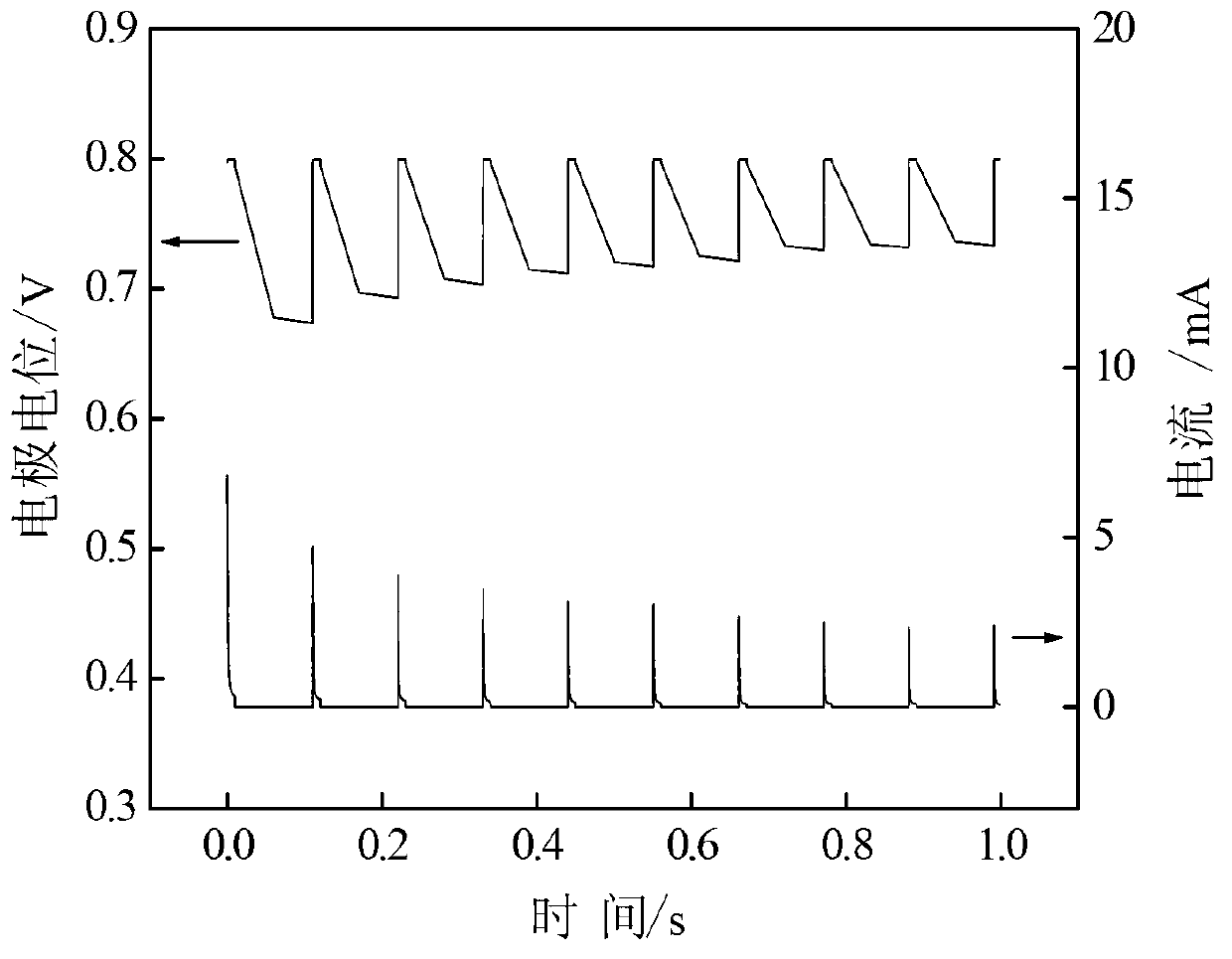
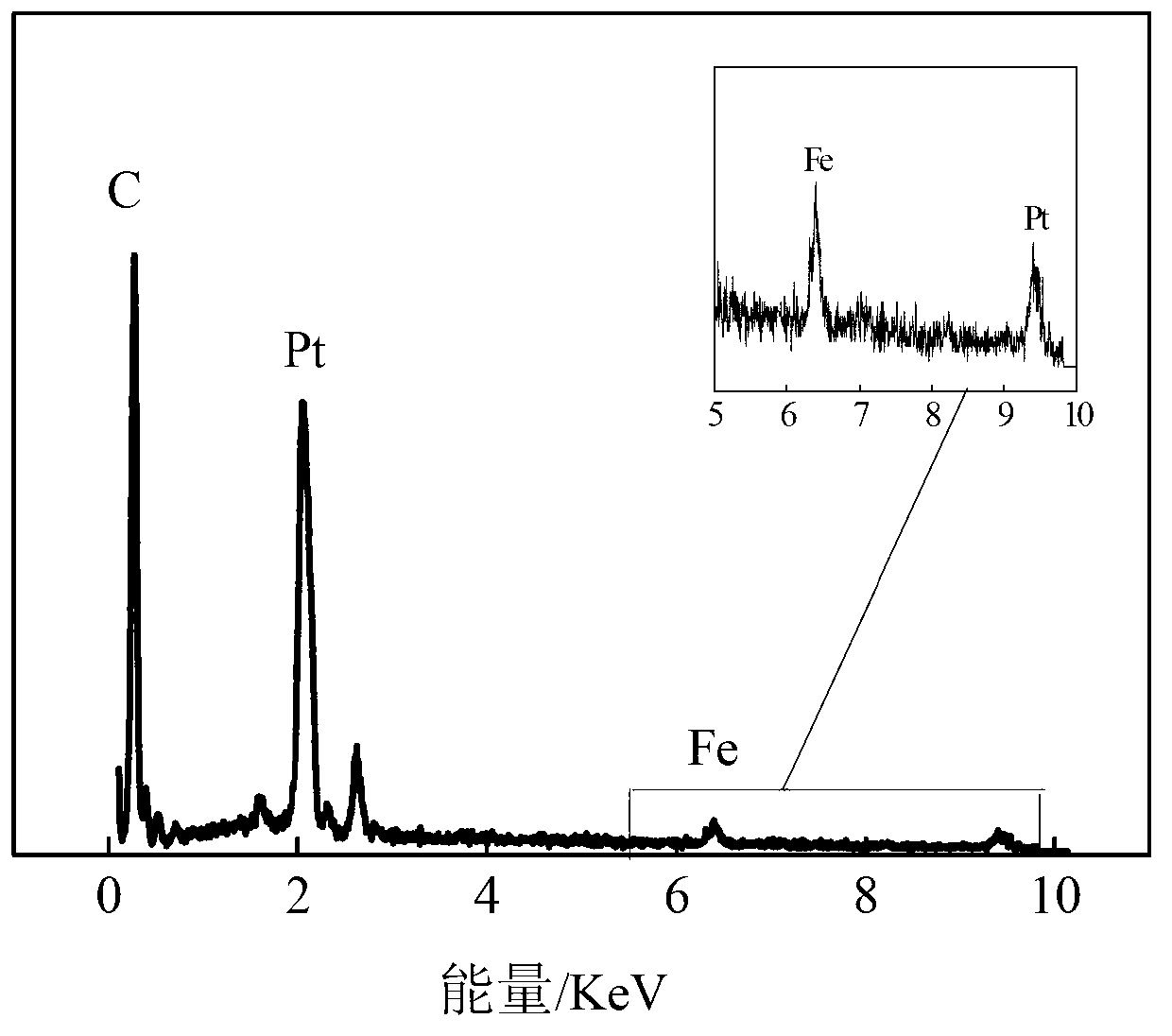
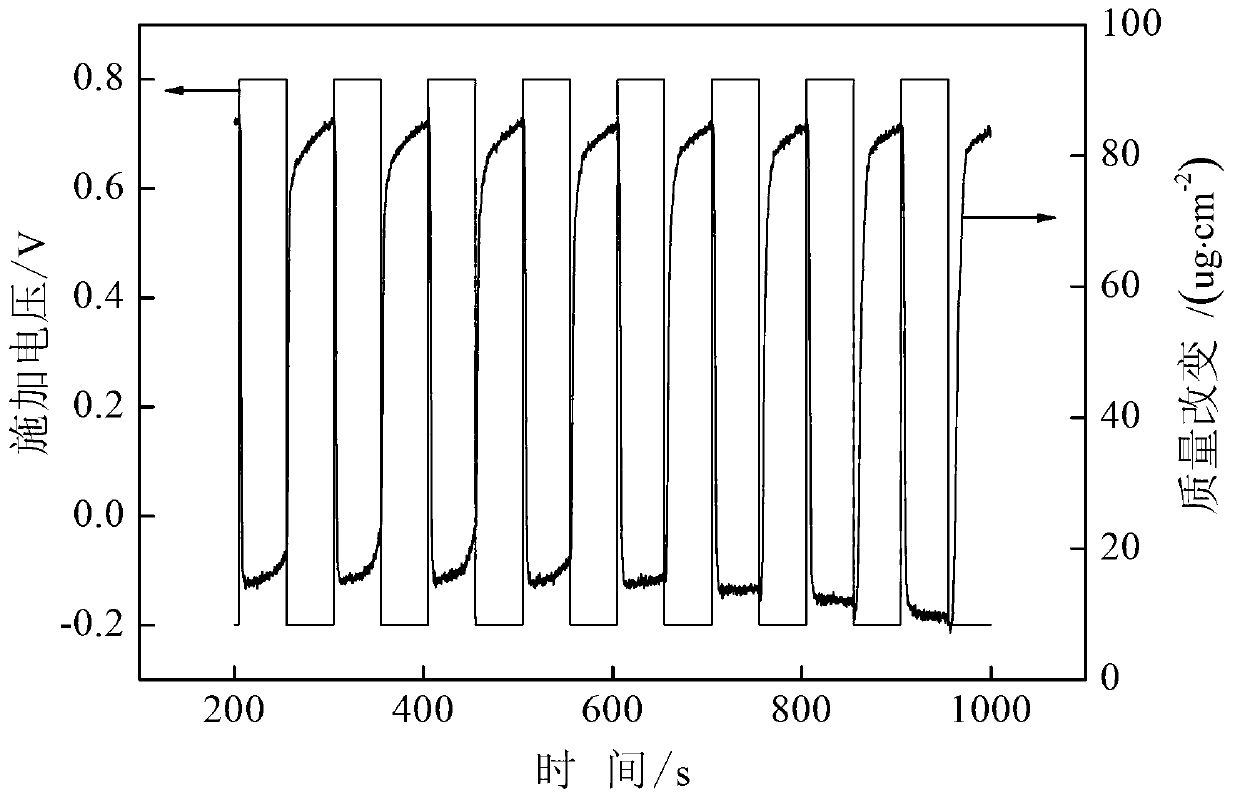
[0064] (7) Using a three-electrode system (the working electrode has an effective area of 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com