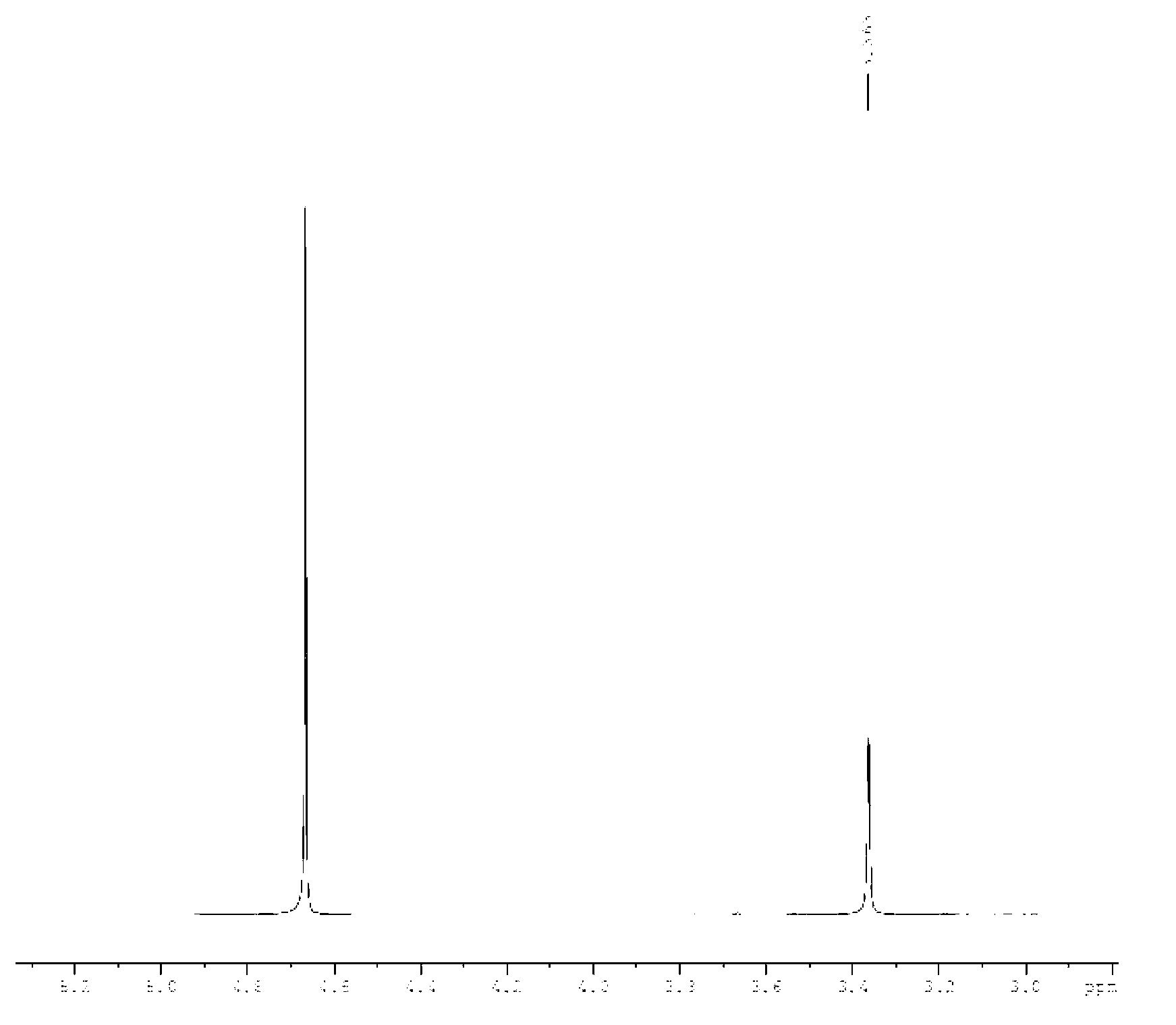
Preparation method of triazine ring
A triazine ring and ring closure reaction technology, which is applied in the field of pharmaceutical intermediates, can solve the problems of inconvenient post-processing, low yield, and high cost, and achieve the effects of easy recrystallization and purification, reduced production costs, and simplified operating processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
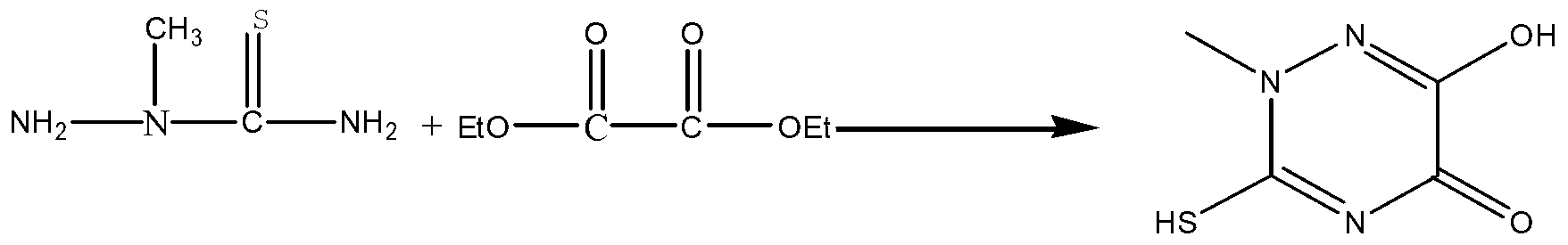
[0026] Specific embodiment one: the preparation method of a kind of triazine ring of this embodiment is carried out according to the following steps:
[0027] 1. Using 2-methylthiosemicarbazide and diethyl oxalate as raw materials, using Lewis acid catalysis, under nitrogen protection, heating and melting, and then performing cyclization reaction, after the cyclization reaction is completed, it is lowered to room temperature to obtain a triazine ring Crude;
[0028] 2. After mixing the crude triazine ring obtained in step 1 with water, heating to reflux, filtering, collecting filtrate, then crystallizing by cooling, filtering, collecting filter cake, drying to obtain triazine ring;
[0029] Wherein, the molar ratio of 2-methylthiosemicarbazide to diethyl oxalate and Lewis acid described in step 1 is 1:(1~1.2):(0.01~0.05); the temperature of cyclization reaction is 120℃~ 150℃, the cyclization reaction time is 2~6h;
[0030] The quality of the water described in the second ste...
specific Embodiment approach 2
[0031] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the Lewis acid described in step 1 is aluminum chloride, ferric chloride, boron trifluoride, titanium tetrachloride or zinc chloride. Others are the same as the first embodiment.
specific Embodiment approach 3
[0032] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the Lewis acid described in Step 1 is aluminum chloride. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com