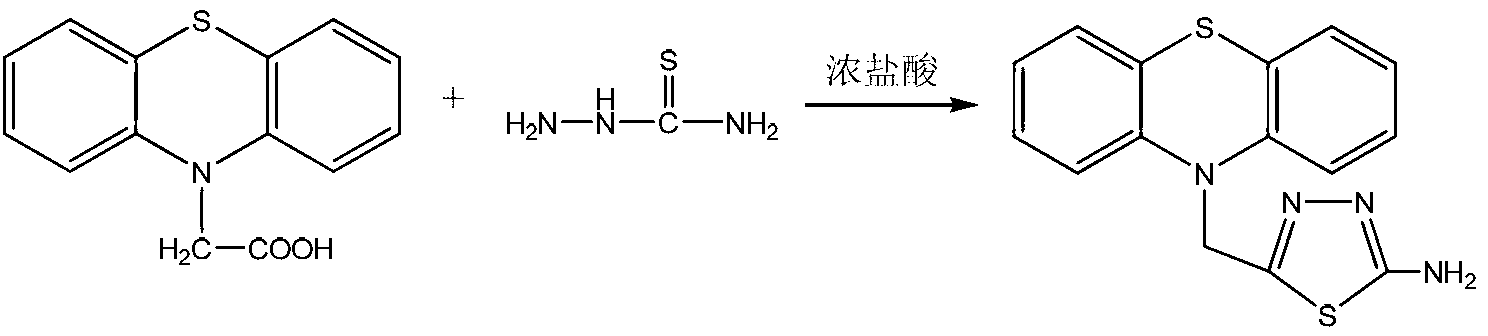
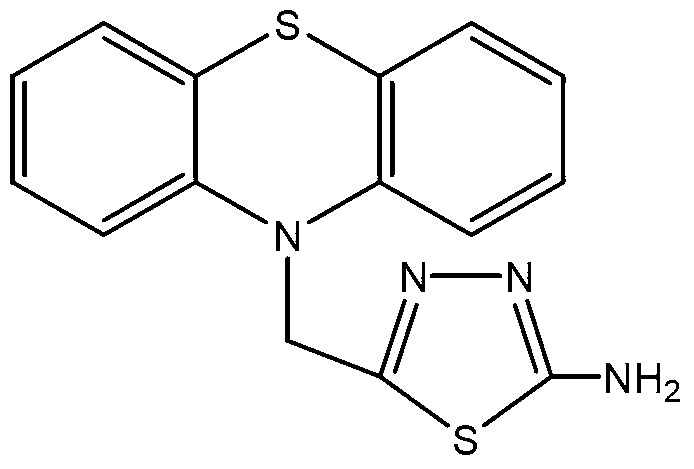
Method for preparing 2-amino-5-(N-phenothiazinyl)methyl-1,3,4-thiadiazole
A technology based on phenothiazine and methylene phenothiazine, which is applied in the direction of organic chemistry, can solve the problems of inconvenient operation, complicated experimental process, and long reaction time, and achieves small secondary pollution, simple experimental steps, and short reaction time. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Add 0.001 mol of N-carboxymethylene phenothiazine (10H-Phenothiazine-10-acetic acid, manufacturer: Shanghai Chemicals Technology Co., Ltd), 0.001 mol of thiosemicarbazide and 10 mL of mass to a dry three-necked flask Concentrated hydrochloric acid with a concentration of 36-38%, turn on the heating and stirring device, and reflux for 4 hours. At this time, TLC detects that the raw material point of N-carboxymethylene phenothiazine disappears, stop the reaction, and obtain a reaction mixture; wherein, TLC The developer is a mixture of ethyl acetate and petroleum ether, and the volume ratio of ethyl acetate and petroleum ether is 1:3;
[0026] 2) After the reaction mixture is cooled to room temperature, adjust the pH value of the reaction mixture to 8 with a 40% aqueous sodium hydroxide solution, then cool the reaction mixture with ice water until solid particles are precipitated, and suction filter to obtain the filter cake Wash with ice water and dry to obtain the cr...
Embodiment 2
[0029] 1) Add 0.001 mol of N-carboxymethylene phenothiazine (10H-Phenothiazine-10-acetic acid, manufacturer: Shanghai Chemicals Technology Co., Ltd), 0.0012 mol of thiosemicarbazide and 11 mL of Concentrated hydrochloric acid with a mass concentration of 36-38%, turn on the heating and stirring device, and reflux for 4 hours. At this time, TLC detects that the raw material point of N-carboxymethylene phenothiazine disappears, stop the reaction, and obtain a reaction mixture; wherein, TLC The developer is a mixture of ethyl acetate and petroleum ether, and the volume ratio of ethyl acetate and petroleum ether is 1:3;
[0030] 2) After the reaction mixture is cooled to room temperature, adjust the pH value of the reaction mixture to 8 with a 40% aqueous sodium hydroxide solution, then cool the reaction mixture with ice water until solid particles are precipitated, and suction filter to obtain the filter cake Wash with ice water and dry to obtain the crude product, which is recry...
Embodiment 3
[0032] 1) Add 0.001 mol of N-carboxymethylene phenothiazine (10H-Phenothiazine-10-acetic acid, manufacturer: Shanghai Chemicals Technology Co., Ltd), 0.0012 mol of thiosemicarbazide and 12 mL of Concentrated hydrochloric acid with a mass concentration of 36-38%, turn on the heating and stirring device, and reflux for 5 hours. At this time, TLC detects that the raw material point of N-carboxymethylene phenothiazine disappears, stop the reaction, and obtain a reaction mixture; wherein, TLC The developer is a mixture of ethyl acetate and petroleum ether, and the volume ratio of ethyl acetate and petroleum ether is 1:3;
[0033] 2) After the reaction mixture is cooled to room temperature, adjust the pH value of the reaction mixture to 8 with a 40% aqueous sodium hydroxide solution, then cool the reaction mixture with ice water until solid particles are precipitated, and suction filter to obtain the filter cake Wash with ice water and dry to obtain the crude product, which is recry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com