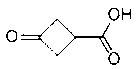
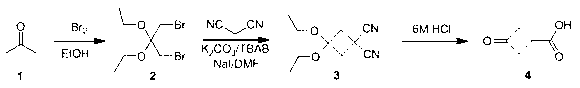Synthesis method of 3-oxocyclobutanecarboxylic acid
A technology of oxocyclobutane carboxylic acid and synthesis method, which is applied in the directions of organic chemistry, nitrile preparation, etc., can solve the problems of difficulty in large-scale production and high reaction temperature, and achieves low synthesis cost, high product purity and simple process route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Synthesis of 1, 3-dibromoacetal
[0033] Acetone (116 g, 2 mol) was added to absolute ethanol (2 L), bromine (640 g, 4 mol) was slowly added dropwise at room temperature, reacted at 20-30°C for 10 hours, and evaporated to dryness directly to obtain a white solid 1 , 493 g of 3-dibromoacetone, yield 85%;
[0034] (2) Synthesis of 3,3-dicyanoethyclobutanone
[0035] Dissolve malononitrile (66 g, 1 mol) in N, N-dimethylformamide (2 L), add potassium carbonate (276 g, 2 mol), sodium iodide (7.5 g, 50 mol) and tetrabutyl Ammonium bromide (16.1g, 50 mmol), stirred at room temperature for 0.5 hours, then heated to 60°C, slowly added dropwise 1,3-dibromoacetone (290 g, 1 mol) in DMF solution (1L), 60°C Stir for 24 hours. After the reaction, cool to room temperature, evaporate DMF under reduced pressure, add toluene (3 L) and water (1.5 L), stir and separate layers, wash the toluene layer with water, dry, filter, evaporate toluene under reduced pressure, and then 144....
Embodiment 2
[0039] (1) Synthesis of 1, 3-dibromoacetal
[0040] Acetone (116 g, 2 mol) was added to absolute ethanol (2 L), bromine (480 g, 3 mol) was slowly added dropwise at room temperature, reacted at 20-30°C for 10 hours, and evaporated to dryness directly to obtain a white solid 1 , 380 g of 3-dibromoacetone, yield 74.4%;
[0041] (2) Synthesis of 3,3-dicyanoethyclobutanone
[0042] Dissolve malononitrile (66 g, 1 mol) in N,N-dimethylformamide (2 L), add potassium carbonate (414 g, 3 mol), sodium iodide (7.5 g, 50 mol) and tetrabutyl ammonium bromide (16.1 g, 50 mmol), stirred at room temperature for 0.5 hours, then warmed up to 60°C, and slowly added dropwise a DMF solution (1 L) of 1,3-dibromoacetal (290 g, 1 mol), 60 Stir at ℃ for 24 hours. After the reaction, cool to room temperature, evaporate DMF under reduced pressure, add toluene (3L) and water (1.5L), stir and separate layers, wash the toluene layer with water, dry, filter, and evaporate toluene under reduced pressure. R...
Embodiment 3
[0046] (1) Synthesis of 1,3-dibromoacetal
[0047]Acetone (116 g, 2 mol) was added to absolute ethanol (2 L), bromine (640 g, 4 mol) was slowly added dropwise at room temperature, reacted at 20-30°C for 16 hours and directly evaporated to dryness to obtain a white solid 1 , 510.5 g of 3-dibromoacetone, yield 88%;
[0048] (2) Synthesis of 3,3-dicyanoethyclobutanone
[0049] Dissolve malononitrile (66 g, 1 mol) in N,N-dimethylformamide (2 L), add potassium carbonate (414 g, 3 mol), sodium iodide (7.5 g, 50 mol) and tetrabutyl ammonium bromide (16.1 g, 50 mmol), stirred at room temperature for 0.5 hours, then warmed up to 60°C, and slowly added dropwise a DMF solution (1 L) of 1,3-dibromoacetal (290 g, 1 mol), 60 Stir at ℃ for 24 hours. After the reaction, cool to room temperature, evaporate DMF under reduced pressure, add toluene (3L) and water (1.5L), stir and separate layers, wash the toluene layer with water, dry, filter, evaporate toluene under reduced pressure, Re-disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com