A kind of rosuvastatin amino acid salt and its preparation method and application
A technology of rosuvastatin and amino acid salt, which is applied in the field of cardiovascular medicine, can solve liver problems and other problems, and achieve the effects of improving water solubility, preferential price and increasing utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
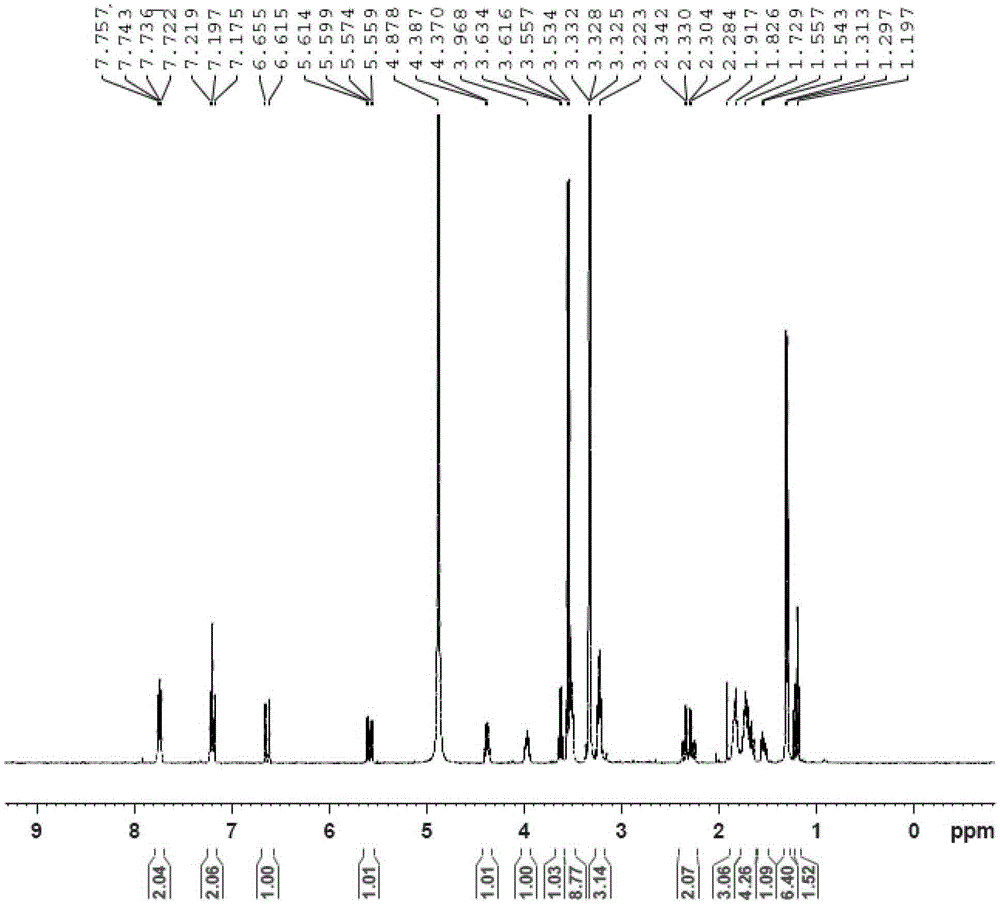
[0077] A kind of rosuvastatin arginine salt, the structural formula after its salification is as follows:
[0078]
[0079] or
[0080]
[0081] or
[0082]
[0083] A preparation method of rosuvastatin arginine salt, the steps are as follows:
[0084] (1) Weigh 2g of rosuvastatin tert-butyl ester (3.46mmol), dissolve in a solvent of 20ml of methanol and 5ml of tetrahydrofuran, and stir to dissolve;
[0085] (2) Add 4ml of 1mol / L hydrochloric acid (4mmol) to the solution in step (1), stir at 30-35°C for 2h, and TLC detects that the reaction is complete;
[0086] (3) Add 1.4ml of 3mol / L sodium hydroxide (4.2mmol) to the solution in step (2), stir and react at room temperature for 1h, evaporate the solvent of the reaction solution to dryness; add water to it, and extract with methyl tert-butyl ether to remove impurities ;
[0087] (4) Adjust the aqueous phase in the solution in step (3) to neutral or weakly acidic with 2mol / L hydrochloric acid, extract with ethyl ac...
Embodiment 2
[0093] A kind of rosuvastatin lysine salt, the structural formula after its salification is as follows:
[0094]
[0095] or
[0096]
[0097] or
[0098]
[0099] A preparation method of rosuvastatin lysine salt, the steps are as follows:
[0100] (1) Weigh 2g of rosuvastatin tert-butyl ester (3.46mmol), dissolve in a solvent of 20ml of methanol and 5ml of tetrahydrofuran, and stir to dissolve;
[0101] (2) Add 4ml of 1mol / L hydrochloric acid (4mmol) to the solution in step (1), stir at 30-35°C for 2h, and TLC detects that the reaction is complete;
[0102] (3) Add 1.4ml of 3mol / L sodium hydroxide (4.2mmol) to the solution in step (2), stir and react at room temperature for 1h, evaporate the solvent of the reaction solution to dryness; add water to it, and extract with methyl tert-butyl ether to remove impurities ;
[0103] (4) Adjust the aqueous phase in the solution in step (3) to neutral or weakly acidic with 2mol / L hydrochloric acid, extract with ethyl acetat...
Embodiment 3
[0109] A kind of rosuvastatin histidine salt, the structural formula after its salification is as follows:
[0110]
[0111] or
[0112]
[0113] or
[0114]
[0115] A preparation method of rosuvastatin histidine salt, the steps are as follows:
[0116] (1) Weigh 2g of rosuvastatin tert-butyl ester (3.46mmol), dissolve it in a solvent of 20ml of methanol and 5ml of tetrahydrofuran, and stir to dissolve;
[0117] (2) Add 4ml of 1mol / L hydrochloric acid (4mmol) to the solution in step (1), stir at 30-35°C for 2h, and TLC detects that the reaction is complete;
[0118](3) Add 1.4ml of 3mol / L sodium hydroxide (4.2mmol) to the solution in step (2), stir and react at room temperature for 1h, evaporate the solvent of the reaction solution to dryness; add water to it, and extract with methyl tert-butyl ether to remove impurities ;
[0119] (4) Adjust the aqueous phase in the solution in step (3) to neutral or weakly acidic with 2mol / L hydrochloric acid, extract with ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com