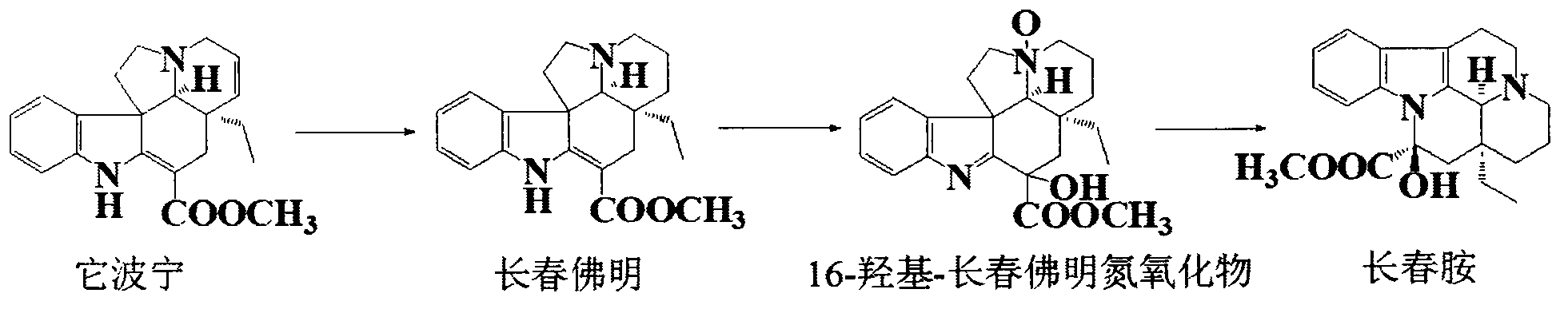
Industrialized semi-synthesis process of vincamine
A technology of vincamine and nitrogen oxides, applied in the direction of organic chemistry, can solve the problems of high production equipment requirements, high cost, and low chemical atom economy, and achieve simple equipment, large economic and social benefits, and environmentally friendly production procedures Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of embodiment 1 Changchun Fermin
[0023] Weigh 5.0 kg of tabonin hydrochloride into a 100 L stainless steel reaction kettle, add 50 L of 75% ethanol, and stir to dissolve at room temperature. Then add 250g of Raney nickel, immediately feed hydrogen, stir after 0.5h, and control the hydrogen pressure to 1.1atm, and react for 8h. After the reaction was completed, nitrogen gas was passed through for 10 minutes, the catalyst was removed by filtration, and the crude product of vinbromin was obtained by concentration. Crystallization with ethyl acetate-ethanol gave 4.8 kg of vinbromin hydrochloride with a purity greater than 99.0%, with a yield of 96%.
Embodiment 216
[0024] Synthesis of embodiment 216-hydroxyl-vinporin oxide
[0025] Take 5.0 kg of vinchun phermin hydrochloride and place it in a 100 L stainless steel reaction kettle, add 50 L of 1,3-dioxane, stir and dissolve at room temperature. Filled with nitrogen protection, protected from light and reacted at room temperature for about 16 hours. The progress of the reaction was detected by TLC, until the Changchun Fermin intermediate basically disappeared, and the nitric oxide intermediate was generated. Add 5% NaHCO to the reaction solution 3 The solution was free of bubbles, and the excess peroxyacid and the generated acid were removed. Separate the organic phase, extract twice with 20 L of dichloroethane, combine the organic phases, dry with 500 g of anhydrous sodium sulfate overnight, and concentrate under reduced pressure to obtain crude 16-hydroxy-vinpophormin nitrogen oxide as an oily substance. Then crystallized with dichloromethane-acetone to obtain 4.0 kg of nitrogen oxid...
Embodiment 3
[0026] The synthesis of embodiment 3 vincamine
[0027] Put 5.0kg of 16-hydroxy-vinfofermin nitrogen oxide in a 100L stainless steel reaction kettle, add 60L of trichloroacetic acid, stir and dissolve at a temperature of about 0°C, then add 3.0kg of triphenylphosphine, and reflux for about 3 hours. The progress of the reaction was detected by TLC until the nitric oxide intermediate disappeared substantially. Cool the reaction solution, add an equal amount of ice water, and wash away triphenylphosphine oxide with dichloroethane. The aqueous phase was then adjusted to pH 8-9 with 1% NaOH, extracted twice with dichloromethane, the combined organic phases were concentrated to 20 L volume, and decolorized by adding activated carbon for 30 min. The organic phase was obtained by filtration, dried overnight with 500 g of anhydrous sodium sulfate, and a certain amount of methanol was added to the mother liquor to crystallize to obtain crude vincamine. The crude product was recrystall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com