Fluorouracil compound, and preparation method and application thereof
A technology of fluorouracil compounds, applied in the field of fluorouracil compounds, their preparation and application, to achieve the effect of simple and feasible route preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
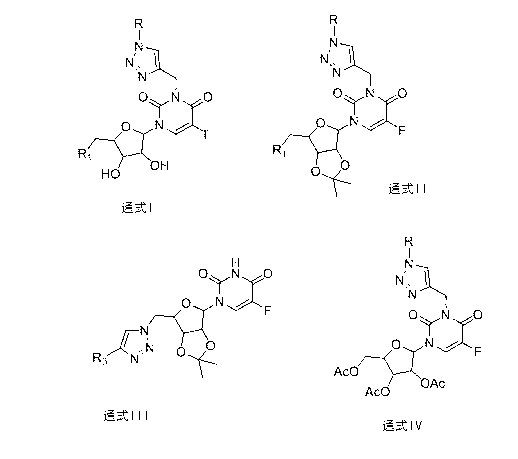
[0050] Preparation of 1-(5′-deoxy-β-D-ribofuranose)-3-(3′-p-fluorobenzyl-1′,2′,3′-triazolyl)-methylene-5-fluorouracil (compound Ⅰ-1 )
[0051] Add 1-(5′-deoxy-β-D-ribofuranose)-5-fluorouracil (8.1mmol, 2g), DMF (20ml), K 2 CO 3 (28.9mmol, 4g), propyne bromide (24.3mmol, 1.9ml), stirred at room temperature, and reacted for 3-5 hours until TLC (254nm, petroleum ether: acetone = 1:1) detected that the raw materials were consumed. After the reaction is completed, add 15ml of distilled water, extract with ethyl acetate (15ml×2), combine the ethyl ester phases, dry with 4g of magnesium sulfate for 3 hours, and vacuum-filter and then rotary evaporate for 40 to obtain a yellow solid, weigh 2.2g after vacuum drying, and set aside. Yield 95.7%.
[0052] Add the above-prepared compound (0.7mmol, 200mg), the mixed solution of tert-butanol and water (9ml, tert-butanol / water=8 / 1), Cu (2.8mmol, 179mg) and CuSO 4 (0.7mmol, 124mg), p-fluorobenzyl azide (2.1mmol, 317mg), react at 50°C fo...
Embodiment 2
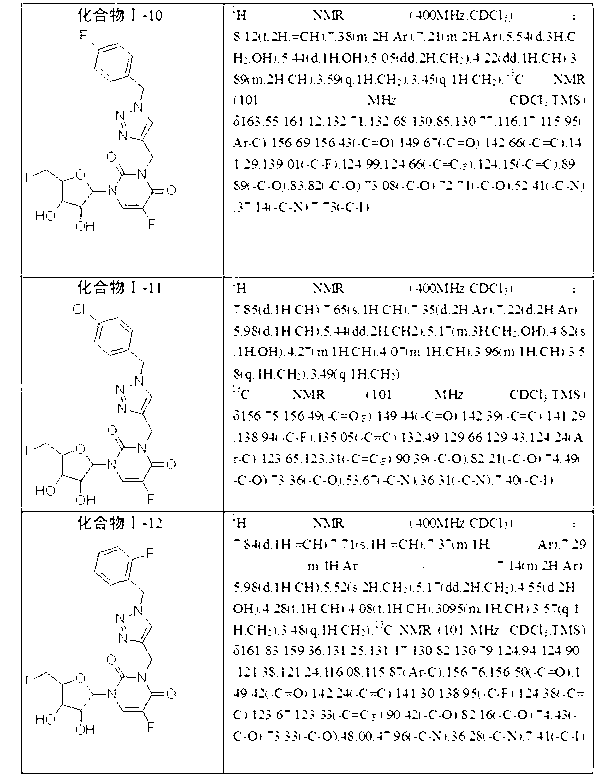
[0054] Compounds I-2 to I-15 were synthesized with reference to Example 1, and the corresponding aromatic methyl azides were used in step (2).
Embodiment 3
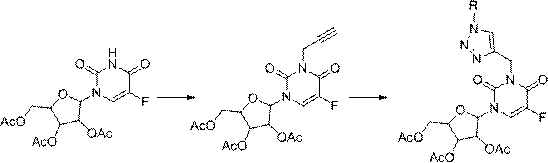
[0056] Synthesis of compound II-1
[0057] Take the compound 1-(2',3'-O-isopropylidene-5'-p-toluenesulfonyl-β-D-ribofuranose)-5-fluorouridine (6.6g, 17mmol) in a 250mL round bottom Into the flask, add acetonitrile 100mL, potassium carbonate (24g, 17mmol), propyne bromide (4mL, 51mmol), stir at room temperature, and react for 5h, TLC (V petroleum ether: V acetone = 3:1) detects that the reaction of raw materials is complete, stop reaction. Diatomaceous earth filter aid, ethyl acetate washed the filter cake, combined organic phase, rotary steamed, added ethyl acetate 80mL, washed with distilled water three times, added magnesium sulfate to dry, suction filtered and rotary steamed to obtain a white solid, weighing 6.3g, Yield 82%, standby.
[0058] Take the compound obtained above (0.297g, 0.66mmol) and place it in a 25mL round bottom flask, add 8mL of tert-butanol and 1mL of water as a mixed solvent, add copper powder (0.128g, 2.0mmol) and copper sulfate (0.083g, 0.33mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com