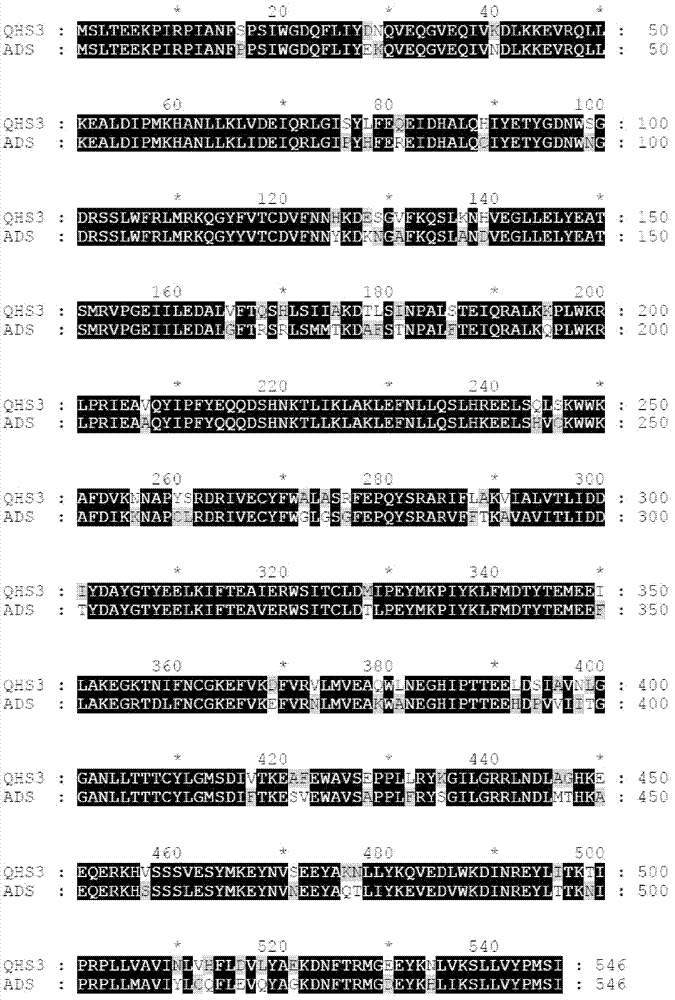
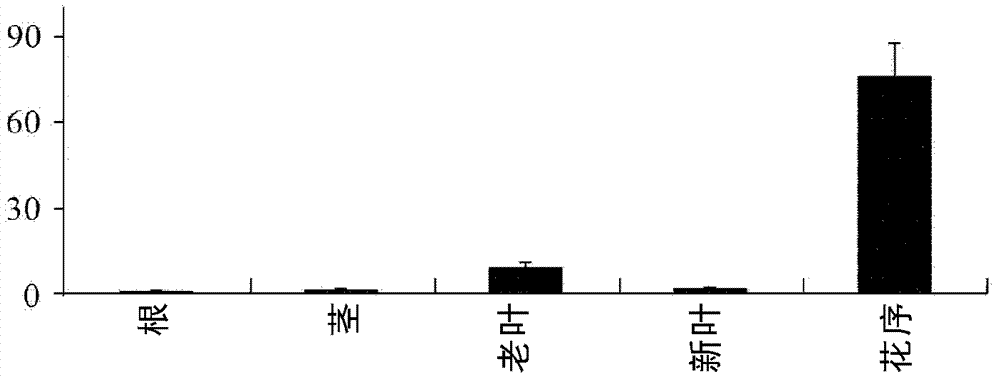
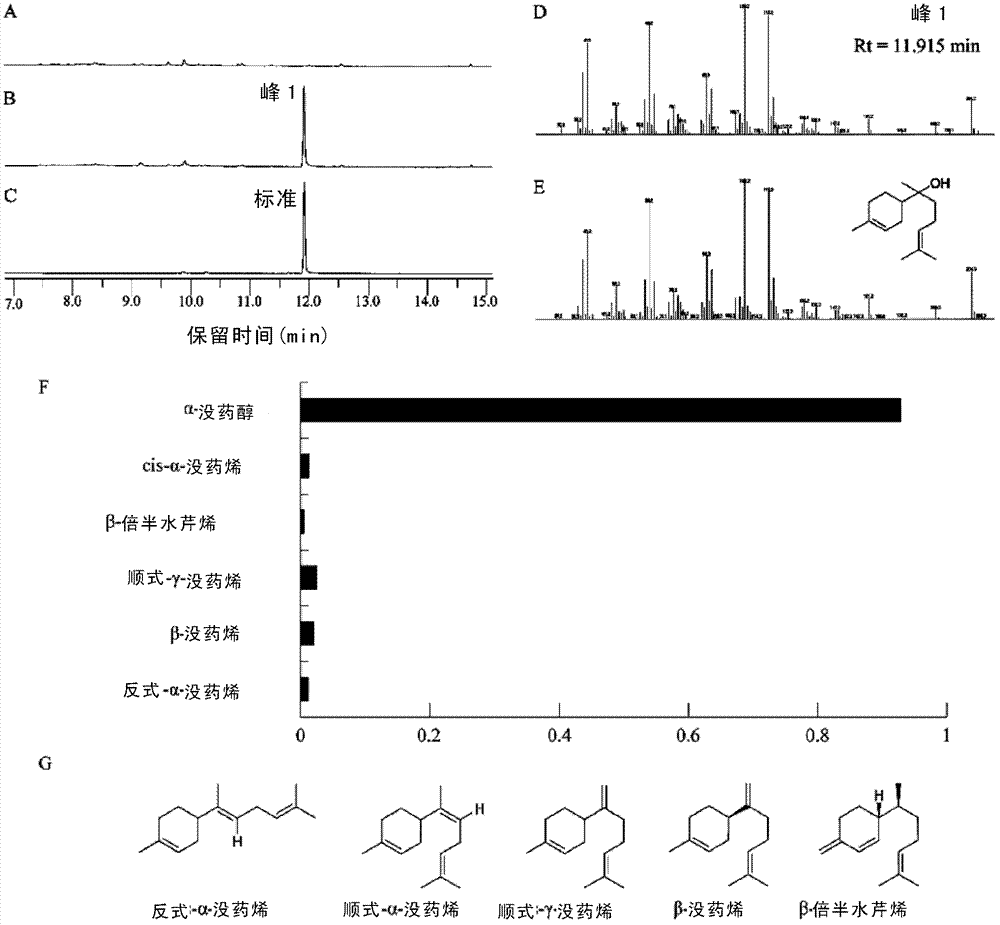
Novel sesquiterpene synthetase and application thereof
An amino acid and sequence technology, applied in the direction of application, lyase, and introduction of foreign genetic material using vectors, can solve unseen problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1, extraction of Artemisia annua total RNA, PCR amplification of target gene QHS3
[0090] A. Extraction of Artemisia annua total RNA
[0091] The Artemisia annua plant material (about 100 mg) was thoroughly ground in liquid nitrogen. Transfer to a 1.5ml centrifuge tube, add 1mL Trizol (Invitrogen, Cat. 15596-018), mix well, and stand at room temperature for 5min. Centrifuge at 12,000rpm for 10min and discard the precipitate. Add 200 μL of chloroform to the supernatant, mix well, and centrifuge at 12,000 rpm for 10 min. Take the supernatant and add 500 μL of isopropanol to precipitate the RNA. Centrifuge at 12,000rpm for 10min, wash the pellet with 70% ethanol, dry in vacuum, dissolve in 20-50μL H 2 O (RNase free). The RNA was properly diluted with 10 mM Tris-HCl (pH 7.5), and the UV absorption value at a wavelength of 200-300 nm was measured. RNA concentration = 40 μg / mL × A 260 ×Dilution factor. A 260 / A 280 Should be between 1.9 and 2.1. PolyAmRNA ...
Embodiment 2
[0111] Embodiment 2, PCR amplification target gene QHS3
[0112] The high-fidelity enzyme KOD-plus DNA polymerase (ToYoBo) was used to amplify the full-length gene sequence of QHS3 (SEQ ID NO: 2) (1641bp). The primer sequences are as follows:
[0113] pETQHS 3-S: 5'-TTTCCATGGCTATGTCTCTTACAGAAGAAAAACCTA-3' (SEQ ID NO: 14),
[0114] pETQHS3-AS: 5'-TTTGGATCCTCATATACTCATAGGATAAACGAGT-3' (SEQ ID NO: 15).
[0115] The PCR reaction conditions were: denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 56°C for 30 s, extension at 68°C for 120 s, amplification for 30 to 35 cycles; incubation at 68°C for 10 min. Keep warm at 4°C.
Embodiment 3
[0116] Example 3, Domain substitution and site-directed mutagenesis of Artemisia annua QHS3
[0117] Using overlap extension PCR technology (Aiyar et al., 1996), multiple domain substitution mutants, single point mutants, double mutants, triple mutants and multiple mutants were successfully constructed. The DNA sequences of these mutants were determined.
[0118] Mutant information and primer sequences are shown in Table 1 (5'-3').
[0119] Table 1
[0120]
[0121]
[0122]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com