Method for preparing spliced artificial bone-filled sustained-release material with treatment effect
A therapeutic effect, artificial bone technology, applied in the field of nano biomedicine, to achieve anti-infective bone regeneration, prolong operation time, and promote bone regeneration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
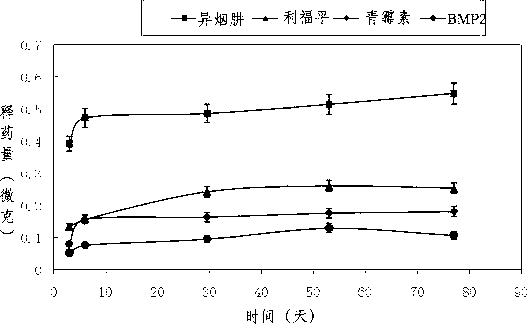
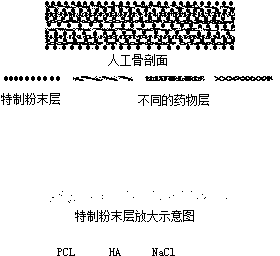
[0030]Accurately weigh 0.24g of PCL with a molecular weight of 80,000, 0.012g of rifampicin powder, add chloroform that is 100 / 7 times the total amount of PCL and rifampicin, and the obtained solution has a voltage of 15kv and a flow rate of 0.5ml / h. Under the condition of spinning for 4 hours, a drug film containing rifampicin was obtained. Accurately weigh 0.24g of PCL with a molecular weight of 80,000, and 0.012g of isoniazid powder, add a mixture of 100 / 7 times the total amount of PCL and isoniazid (chloroform:DMF=9:1), and the obtained solution is Spinning for 4 hours under the conditions of 15kv and flow rate of 0.5ml / h, the drug film containing isoniazid was obtained. The powder is mixed and ground according to the ratio of TCP:HA:PCL:NaCl=(0.4:0.6:1:8) to obtain a special powder. Accurately weigh 0.1g of the special powder and place it in the mold, add isoniazid drug film matching the size of the mold, add 0.1g of the special powder, add rifampicin drug film matching ...
Embodiment 2
[0032] Accurately weigh chitosan 0.24g, penicillin powder 0.012g, add chitosan and penicillin 100 / 7 times the total amount of chloroform, the solution obtained is 12kv at a voltage, and the flow rate is 1ml / h for spinning for 3h. A drug film containing penicillin was obtained. The powder is mixed and ground according to the ratio of BMP2:HA:PCL:NaCl=(0.4:0.6:1:8) to obtain a special powder. Accurately weigh 0.1g of the special powder and place it in the mold, add isoniazid drug film that matches the size of the mold, add 0.1g of the special powder, add the drug film that matches the size of the mold, add 0.1g of the special powder, and add the film that matches the size of the mold Add 0.1g of special powder, add a film that matches the size of the mold, add 0.1g of special powder, add a film that matches the size of the mold, add 0.1g of special powder, add a film that matches the size of the mold, add Special powder 0.1g. Place it together with the mold at 80°C for 20 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com