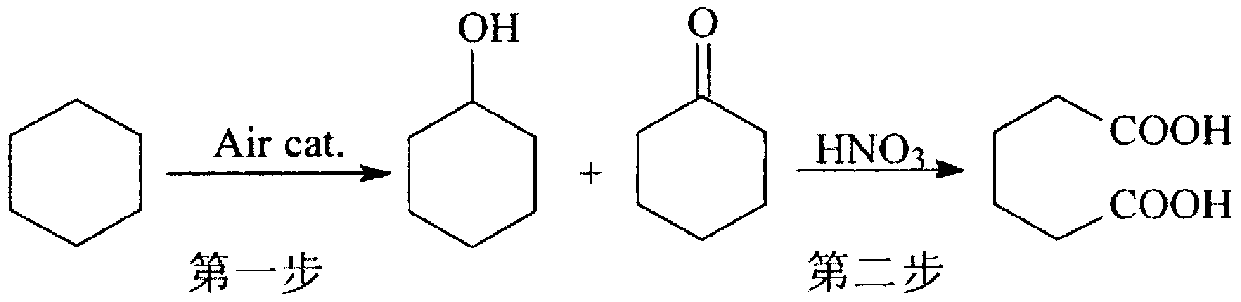
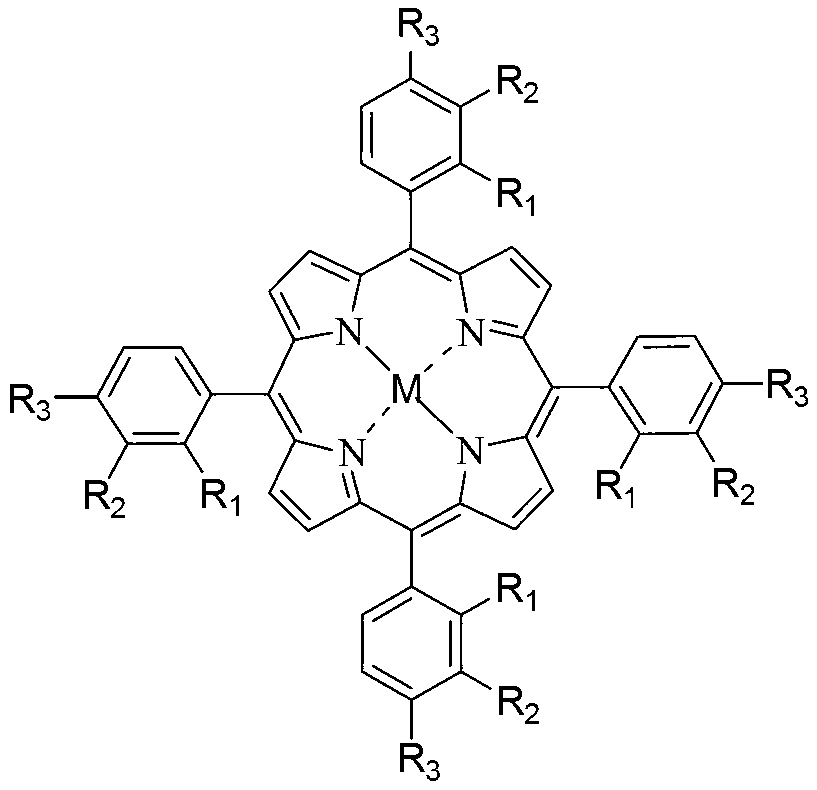
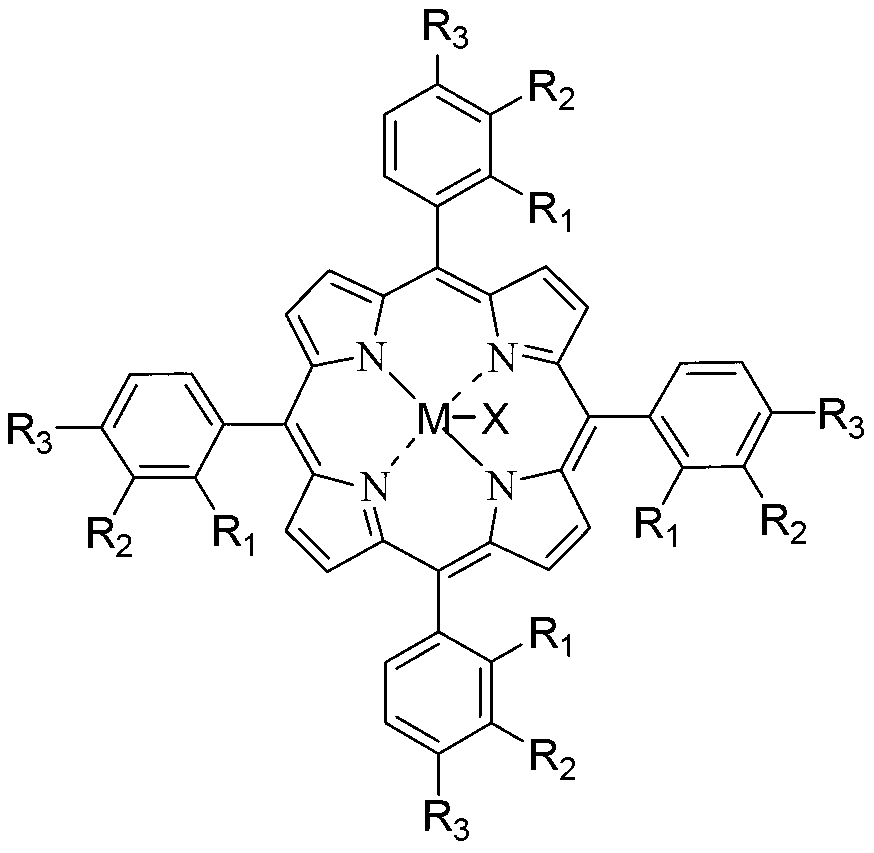
Method for preparing adipic acid through co-catalytic oxidation of six-carbon oxygenated compound and cyclohexane
A technology of cyclohexane and compound, applied in the field of preparation of adipic acid, can solve the problems of difficult catalyst recovery, environmental pollution, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Add 600g cyclohexane and 200g solvent acetic acid in a 1.2L batch oxidation reactor, and add 0.6mg metal phthalocyanine (R with general formula (IV) structure 1 = H, R 2 =CH 3 CH 2 , M=Co) as the catalyst, 36g of cyclohexyl hydroperoxide as the co-oxidant, and then 1.2MPa of oxygen was continuously fed into the reactor to maintain the pressure in the reactor at 1.0MPa and the temperature at 153°C to continuously stir the reactants, and stop after 3.5 hours Reaction to obtain the reaction product, cooling, crystallization, filtration and washing to obtain the adipic acid product. The analysis results of gas chromatography and liquid chromatography showed that the conversion rate of cyclohexane was 17.6%, and the selectivity of adipic acid in the final oxidation product diacid was 93.2%.
Embodiment 2
[0074] Add 600g cyclohexane and 200g solvent acetic acid in a 1.2L batch oxidation reactor, and add 5.4mgMnO 2 As a catalyst, 18g of cyclohexyl hydroperoxide is used as a co-oxidant, and then 0.75MPa of oxygen is continuously introduced to maintain the internal pressure of the reactor at 0.6MPa and the temperature at 125°C to continuously stir the reactants. After 4 hours, the reaction was stopped to obtain a reaction product, which was cooled, crystallized, filtered and washed to obtain adipic acid product. The analysis results of gas chromatography and liquid chromatography showed that the conversion rate of cyclohexane was 14.8%, and the selectivity of adipic acid in the final oxidation product diacid was 91.4%.
Embodiment 3
[0076] Add 600g hexanaphthene and 200g solvent acetic acid in a 1.2L batch oxidation reactor, and add 4.8mg metalloporphyrin (R with general formula (I) structure 1 =R 2 =R3 =H, M=Co) as the catalyst, 18g of cyclohexyl hydroperoxide as the co-oxidant, and then 0.75MPa of oxygen was continuously introduced, and the reactants were continuously stirred under the conditions of maintaining the pressure in the reactor at 0.6MPa and the temperature at 125°C. After 4 hours, the reaction was stopped to obtain a reaction product, which was cooled, crystallized, filtered and washed to obtain adipic acid product. The analysis results of gas chromatography and liquid chromatography showed that the conversion rate of cyclohexane was 13.8%, and the selectivity of adipic acid in the final oxidation product diacid was 88.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com