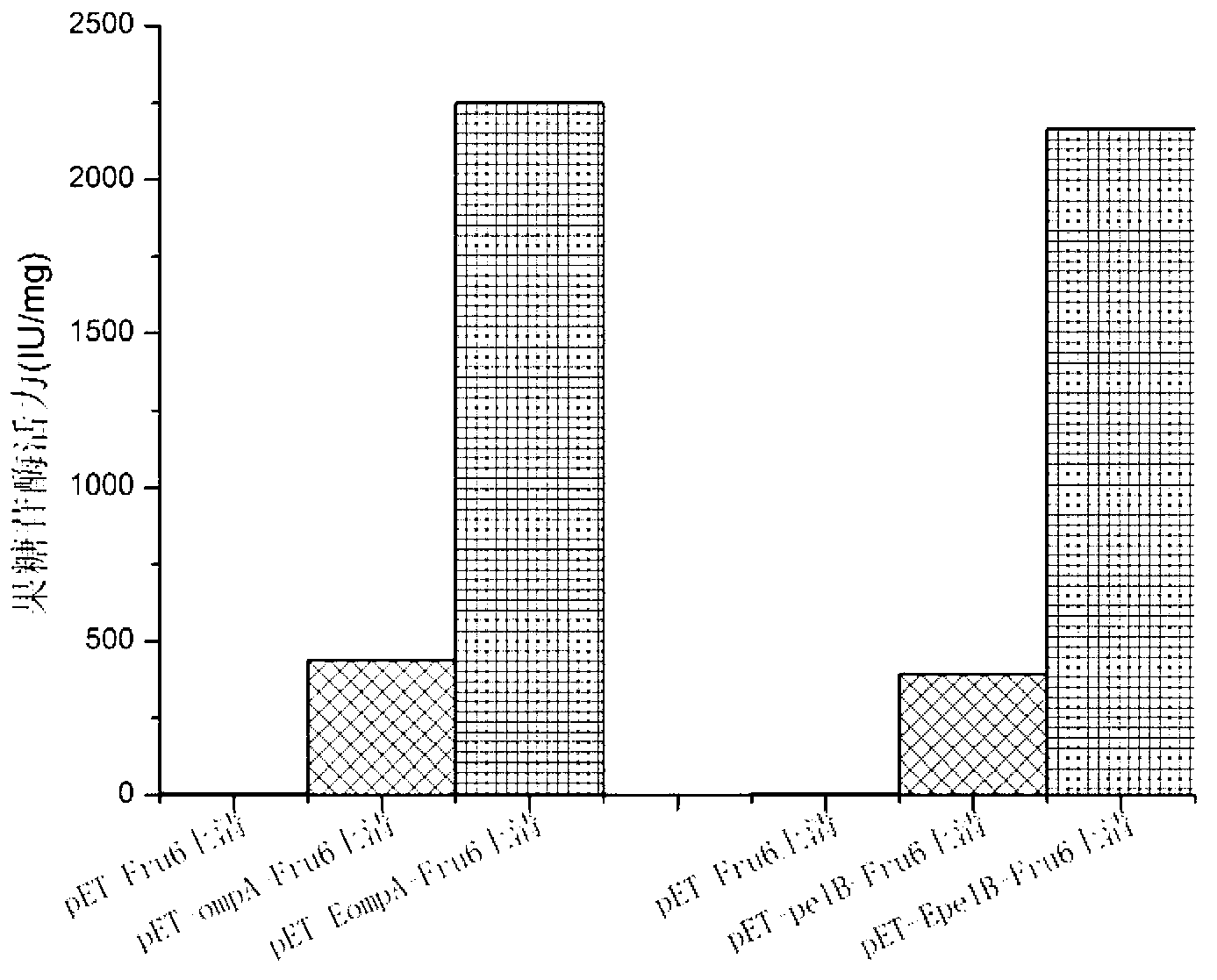Small peptide strengthening mold sequence of strong secretory signal peptide and application thereof
A secretory, signal peptide technology, applied in the field of protein engineering and genetic engineering, to achieve the effect of enhancing the secretion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
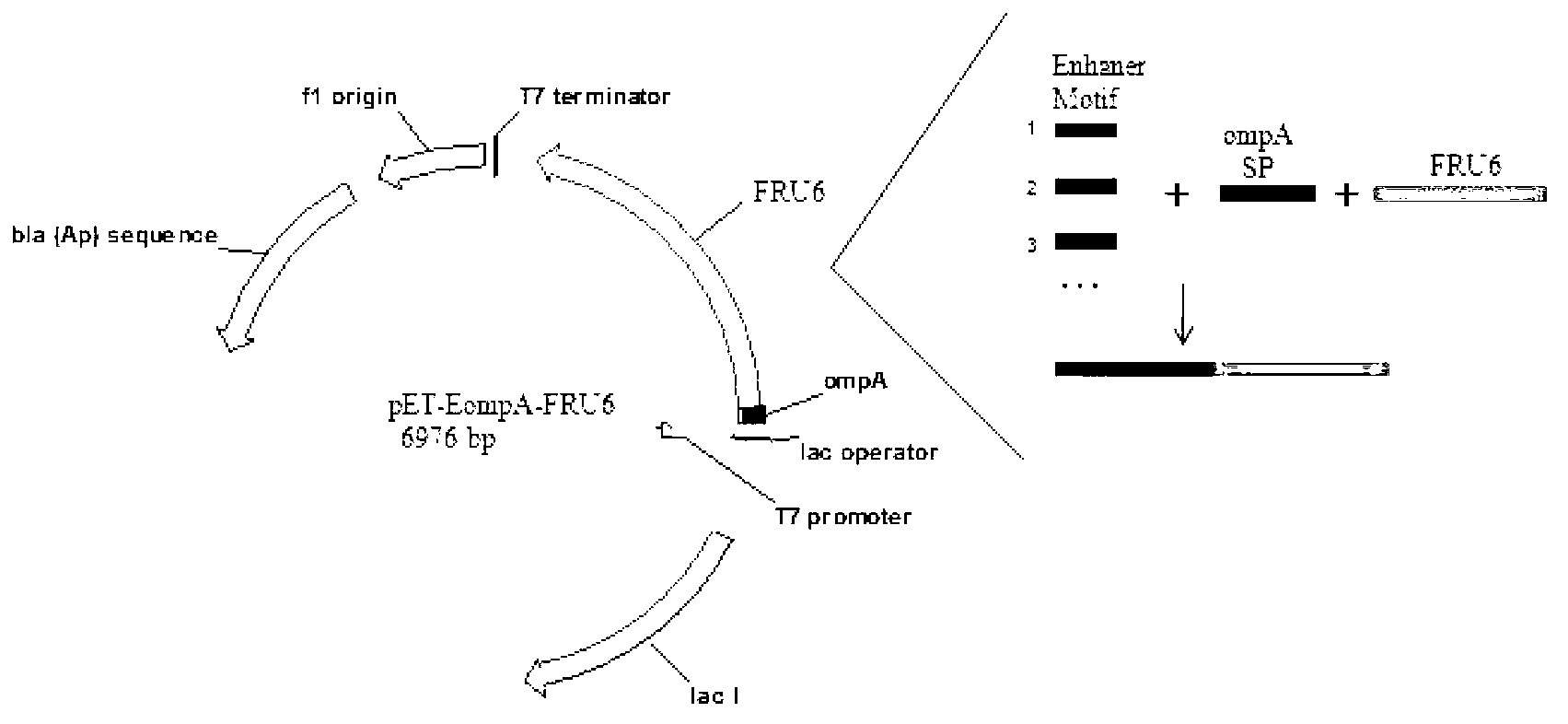
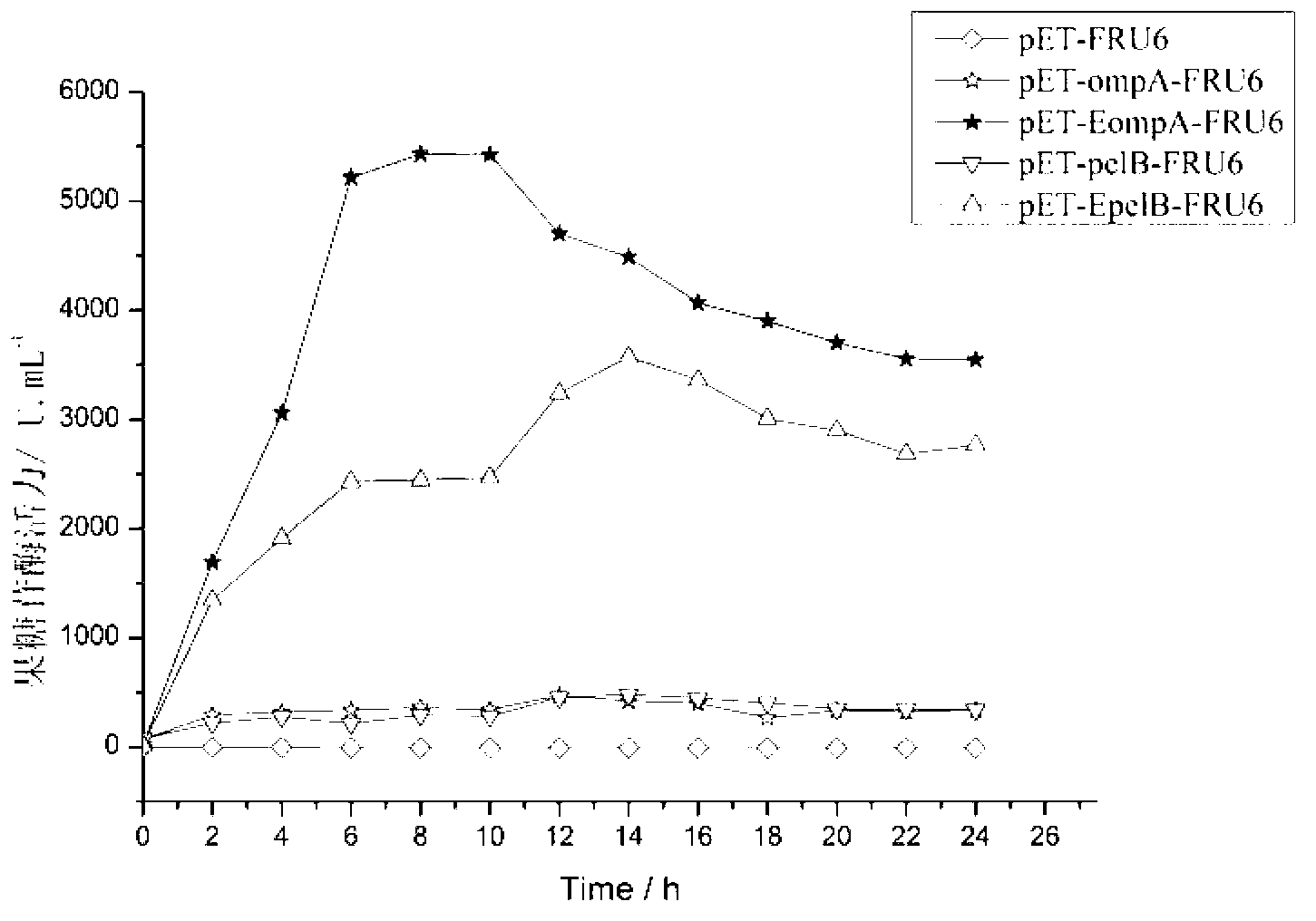
[0060] Example 1. Application of Small Peptide Enhanced Motif in Secreted Expression of Fructosesidase
[0061] First, the source of the fructosidase Fru6 gene described in this example is Arthrobacter arilaitensis NJEM01, which is the inventor's prior Chinese patent application CN102732456A, and the preservation number of this strain is: CCTCC NO: M2012155.
[0062] All primers involved in this example were synthesized by Yingjun Company, see Table 1. The following primer numbers are uniformly represented by "P" plus a sequence number, for example, primer No. 45 in Table 2, its code is P45, and its number in the sequence table is SEQ ID NO:45.
[0063] The nucleotide sequences of primers and synthetic sequences required for table 1 embodiment 1
[0064]
[0065] Note: The underlined part in the table is the restriction enzyme cutting site.
[0066] (1) Acquisition of the fructosidase FRU6 gene: Using the genome of Arthrobacter arilaitensis NJEM01 as a template, the gene ...
Embodiment 2
[0088] Example 2 Application of Small Peptide Enhancing Motif in Glucanase Secretion and Expression
[0089] (1) Construction of expression vectors with small peptide-enhanced motifs added before different signal peptides at the N-terminal of glucanase BGL
[0090] Same as Example 1, in this example, the fructosidase in Example 1 is replaced by the glucanase gene.
[0091] Among them, the source of the sequence of glucanase BGL: Bacillus subtilis subsp.subtilis6051-HGW, GenBank sequence number: CP003329.1, range: 4011849 to 4012490.
[0092] The gene fragment of glucanase BGL was amplified by PCR reaction with primers P79 and P80, and was prepared into T vector pMD-T-BGL. Overlapping PCR primers P81-P84 or P85-P88 were fused with glucanase BGL and signal peptide ompA or pelB, respectively, and both P84 and P88 had EcoRI restriction sites. P89-P96 upstream primers are used in combination with downstream primers P84 or P88 to design different enhancing motifs to be fused in fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com