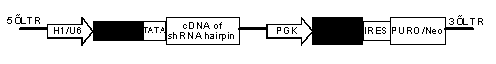
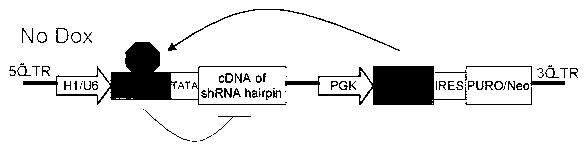
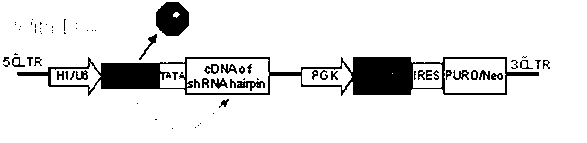
Inducible shRNA (short hairpin ribonucleic acid) lentiviral expression vector and construction method and application thereof
A technology of expression vector and lentivirus, which is applied in the field of functional genomics research and can solve problems such as difficult operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The inducible shRNA lentiviral expression vector specifically inhibits the oncogene PIK3CA, which requires the following steps:
[0068] 1. Construction of inducible shRNA lentiviral expression vector:
[0069] The shRNA expression vector provided by the present invention can be obtained by multi-step subcloning and transforming the lentiviral vector pLKO (from Sigma) containing the NEO selection gene. First, the restriction enzyme xho 1 and not 1 Excision of the constitutive U6-starting polymerase III promoter from the lentiviral vector. The resulting vector backbone was purified and recovered by agarose gel electrophoresis and QIAquick gel extraction kit (Qiagen). The tetracycline (Tet)-inducible polymerase III promoter U6 / TRE was chemically synthesized (SEQ ID 2), and used as a template to amplify and introduce the tetracycline-responsive element TRE sequence and TATA box using polymerase chain reaction (PCR) technology Sequences and Restriction Enzymes xho 1...
Embodiment 2
[0090] The inducible shRNA lentiviral expression vector specifically inhibits the oncogene KRAS gene, which requires the following steps:
[0091] 1. Construction of inducible shRNA lentiviral expression vector:
[0092] The shRNA expression vector of the present invention can be obtained by multi-step subcloning and transforming the lentiviral vector pLKO (from Sigma) containing the PURO selection gene (SEQ ID 5). First, the restriction enzyme xho 1 and not 1 Excision of the constitutive U6-starting polymerase III promoter from the lentiviral vector. The resulting vector backbone was purified and recovered by agarose gel electrophoresis and QIAquick gel extraction kit (Qiagen). The tetracycline (Tet)-inducible polymerase III promoter H1 / TRE was chemically synthesized (SEQ ID 1), and used as a template to amplify and introduce the tetracycline response element TRE sequence, TATA box sequence and restriction endonuclease xho 1 and not 1 Restriction site. product by ...
Embodiment 3
[0112] Inducible shRNA lentiviral expression vector for high-throughput drug target screening
[0113] The process of generating lentivirus from the 14K shRNA pool is as follows:
[0114] 5 L of virus was aliquoted and frozen at -80°C in preparation for infection experiments. The process of the infection experiment was carried out under the following conditions.
[0115]In order to determine the volume of virus that can produce a multiplicity of infection of 0.3-0.5 for each cell line, the virus was divided into 6 samples of different volumes (0-400 μL), and the cells were infected by the titer method, and the post-infection The cells were cultured in puromycin-containing and puromycin-free environments. Infected cells were filtered with a 40 μm cell strainer (manufactured by BD Falcon) before large-scale infection.
[0116] The infection experiments were divided into four groups, and each group of experiments was first divided into 3.7×10 7 Each cell was resuspended in 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com