HPV16L1-h protein and coding gene and application thereof
A technology of L1 protein, pmv-05-hpv16l1-h, applied in application, gene therapy, genetic engineering, etc., can solve the problems of high production cost, inability to use, low expression level, etc., and achieve particle integrity, good safety, The effect of excellent immune characteristics and biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The gene sequence of embodiment 1 coding HPV16L1-h protein is optimized
[0051] According to the nucleotide sequence of the L1 protein of the highly pathogenic HPV16 strain popular in my country, the vector software was used to optimize the design of the HPV16L1 gene sequence according to the most preferred codons of Hansenula to improve its expression in Hansenula cells expression volume. The nucleotide sequence of the gene encoding HPV16L1-h protein provided by the present invention is the sequence without the yeast secretion signal peptide or the transcription termination signal recognized by the yeast. The codons of the gene encoding HPV16L1-h protein in the present invention use the most preferred codons of Hansenula. For codon usage frequencies in Pichia angusta see http: / / www.kazusa.or.jp / codon / . In order to prevent the GC content of the translated mRNA from being too high and the secondary structure of the mRNA from affecting the translation efficiency, the ...
Embodiment 2
[0052] The construction of embodiment 2 recombinant expression vector PMV-05-HPV16L1-h
[0053] 1. Construction process of expression vector PMV-05:
[0054] The expression vector PMV-05 of the present invention consists of 6 parts: promoter (MOX-P), terminator (MOX-T), replicon HARS, ura3 gene, ColE1 replicon, Amp resistance gene.
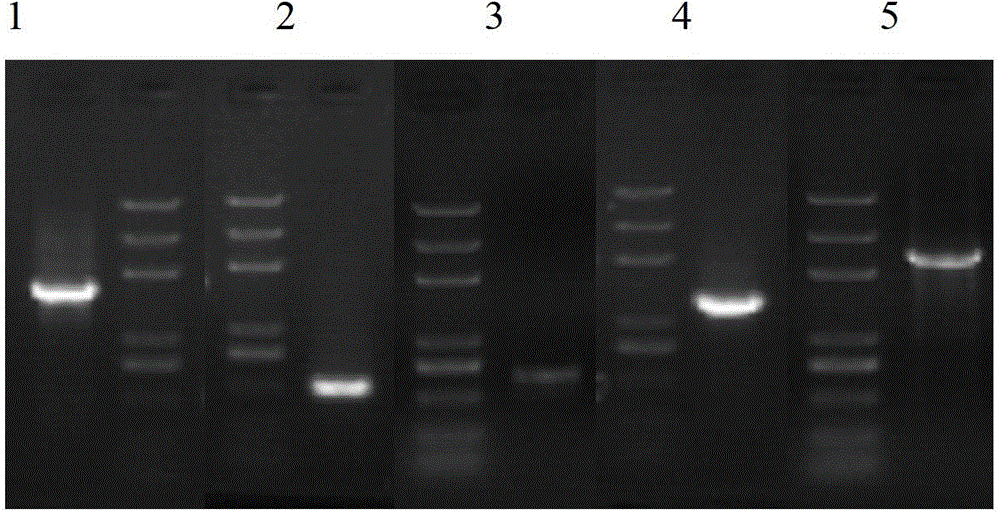
[0055] Using yeast genomic DNA as a template, primers MOXP-F (see SEQ ID NO.3 for sequence), MOXP-R (see SEQ ID NO.4); MOXT-F (see SEQ ID NO.5), MOXT-R respectively (see SEQ ID NO.6); HARS-F (see SEQ ID NO.7), HARS-R (see SEQ ID NO.8); Ura3-F (see SEQ ID NO.9), Ura3-R (see SEQ ID NO.10) was amplified by PCR to extract genes MOXP, MOXT, HARS, and Ura3. Using the PBR322 plasmid (purchased from Treasure Bioengineering Dalian Co., Ltd., item number: D3050) as a template, and using primers Amp+ColE1-F (see SEQ ID NO.11), Amp+ColE1-R (see SEQ ID NO.12) for Amplify by PCR and transfer the gene Amp+ColE1. The PCR reaction system is as follows: 10 μl o...
Embodiment 3
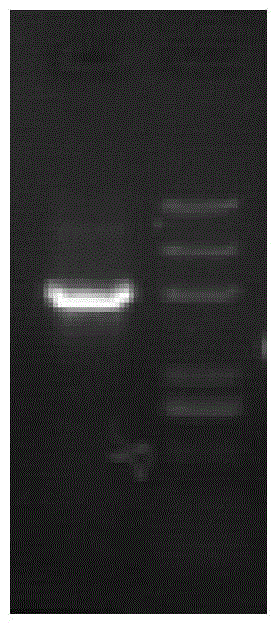
[0061] Example 3 Induced Expression and Detection of HPV16L1 Strain H Expressed by Hansenula
[0062] The recombinant expression vector PMV-05-HPV16L1-h was transformed into Hansenula ATCC26012 uracil-deficient host cells by electroporation (the wild-type host bacteria came from ATCC26012, and the ATCC26012 uracil-deficient host cells were obtained by gene knockout method).
[0063] ATCC26012 uracil-deficient host cells were obtained: the G418 resistance gene sequence was obtained from the Pichia pastoris expression vector pPIC9K (purchased from inritrogen), and the Hansenula Ura3 gene sequence was obtained from Gene bank. Primers P1, P2, P3, P4, P5, and P6 were designed according to the Ura3 and G418 gene sequences, and their nucleotide sequences are shown in SEQ ID NO.13-18, respectively.
[0064] Using the genomic DNA of Hansenula wild-type host strain ATCC26012 as a template, the 5' end gene fragment of Ura3 was obtained by PCR amplification with primers P1 and P2 respecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com