Method for preparing pharmaceutical dextran and levulose by bi-enzyme immobilization coupled multistage membrane separation
A technology for immobilizing dextran and enzymes, which is applied in the direction of fermentation, etc., can solve problems such as low yield of target products, complicated separation of subsequent products, and influence on the safe use of medicinal dextran, so as to facilitate industrialization and application, reduce production costs, and reduce production costs. The effect of high material conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
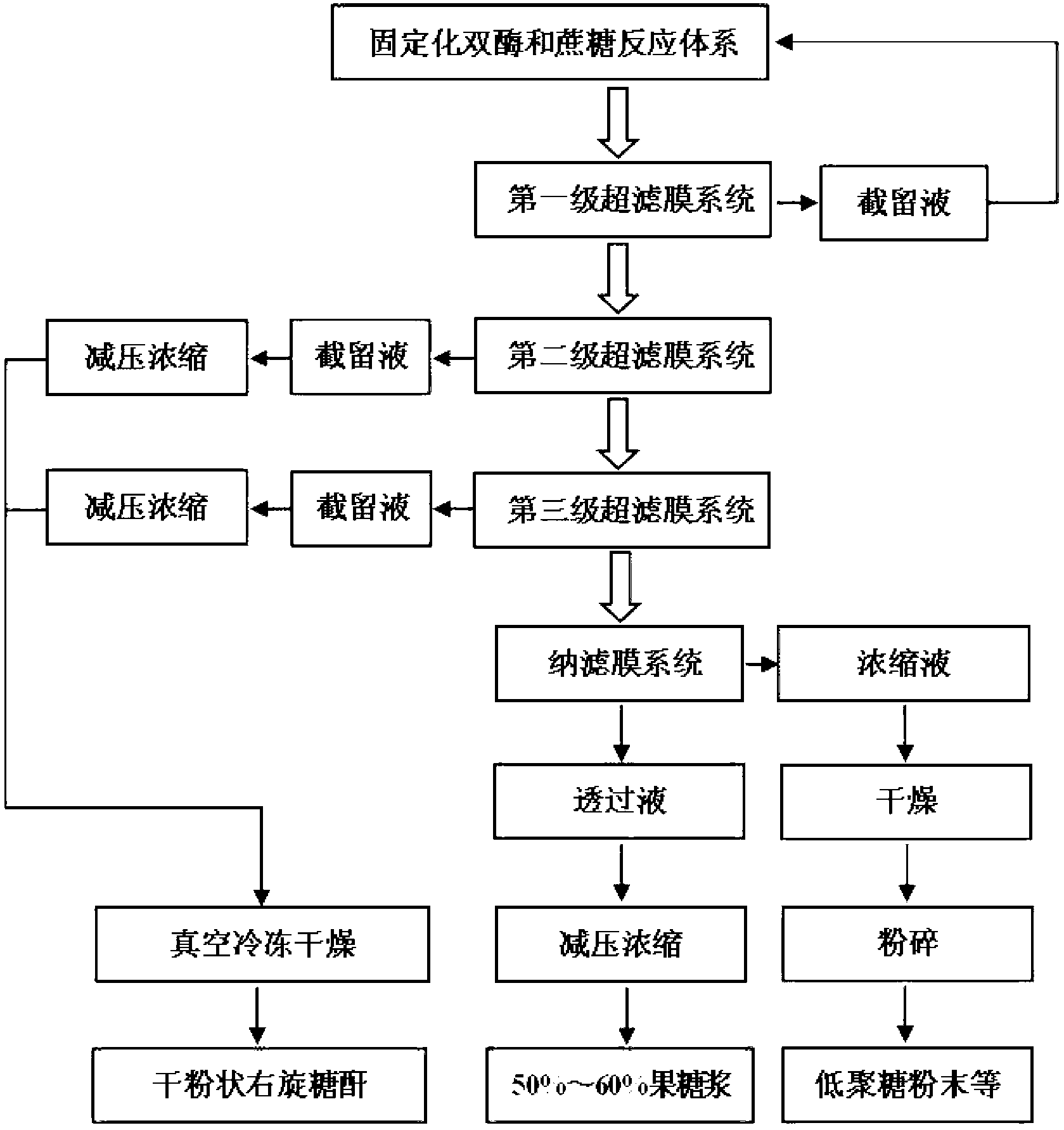
Image
Examples
Embodiment 1
[0022] Dextransucrase was fermented by Leuconostoc enterococci, the fermentation broth was centrifuged at 12,000r / min and 4°C for 20min, and the supernatant was obtained. The enzyme activity was detected by DNS method. Dextranase is derived from a commercial enzyme preparation (Amano Amano Enzyme Trading (Shanghai) Co., Ltd.), and the enzyme activity of dextranase is measured by the Hanes method combined with the actual situation of the present invention and the measurement conditions are slightly modified. It was determined that the enzyme activity of dextran sucrase before immobilization was 8.4U / mL, and the enzyme activity of dextranase was 22,000U / mL.
[0023] The double-enzyme immobilization method is to mix dextransucrase and dextranase with the ratio of enzyme activity of 10:1 (the final concentration of immobilized dextransucrase is 0.6U / mL) and pasteurized sodium alginate solution, seaweed The final concentration of sodium bicarbonate solution was 2.5% (w / v, g / 100mL)....
Embodiment 2
[0026] The acquisition of dextran sucrase and dextranase and the determination of enzyme activity are the same as in Example 1.
[0027] The double-enzyme immobilization method is to mix dextran sucrase and dextranase with the ratio of enzyme activity of 10:1 (the final concentration of immobilized dextran sucrase is 0.8U / mL) and pasteurized sodium alginate solution, seaweed The final concentration of sodium bicarbonate solution was 4.0% (w / v, g / 100mL). The mixed solution was degassed at 4°C for 2 hours, then vacuumed and degassed for 15 minutes, and then the mixed solution was dripped into 2 % sterile calcium chloride solution to prepare immobilized pellets, the immobilized pellets were rinsed with sterile water, and then put into 0.4% (v / v, mL / mL) glutaraldehyde solution for cross-linking After 45 minutes, they were taken out, washed with sterile water, and part of the immobilized pellets were taken to measure the enzyme activity of dextran sucrase and dextranase respectivel...
Embodiment 3
[0029] The acquisition of dextran sucrase and dextranase and the determination of enzyme activity are the same as in Example 1.
[0030] The double-enzyme immobilization method is to mix dextran sucrase and dextranase with the ratio of enzyme activity of 100:1 (the final concentration of immobilized dextran sucrase is 0.8U / mL) and pasteurized sodium alginate solution, seaweed The final concentration of sodium bicarbonate solution was 3.0% (w / v, g / 100mL). The mixed solution was degassed at 4°C for 2 hours, then vacuumed and degassed for 15 minutes, and then the mixed solution was dripped into 2 % sterile calcium chloride solution to prepare immobilized pellets, the immobilized pellets were rinsed with sterile water, and then cross-linked in 0.7% (v / v, mL / mL) glutaraldehyde solution After 40 minutes, remove them, wash them with sterile water, take some immobilized pellets to measure their dextransucrase and dextranase activities, and store the rest at 4°C for later use. The res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com