Environment-friendly preparation method of pentaerythritol acrylic ester and derivate
A technology of pentaerythritol acrylate and derivatives, which is applied in the field of acrylate synthesis, can solve the problems of poor catalyst cycle performance, complicated post-treatment process, and low product yield, so as to avoid discharge of salty wastewater, easy recycling, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
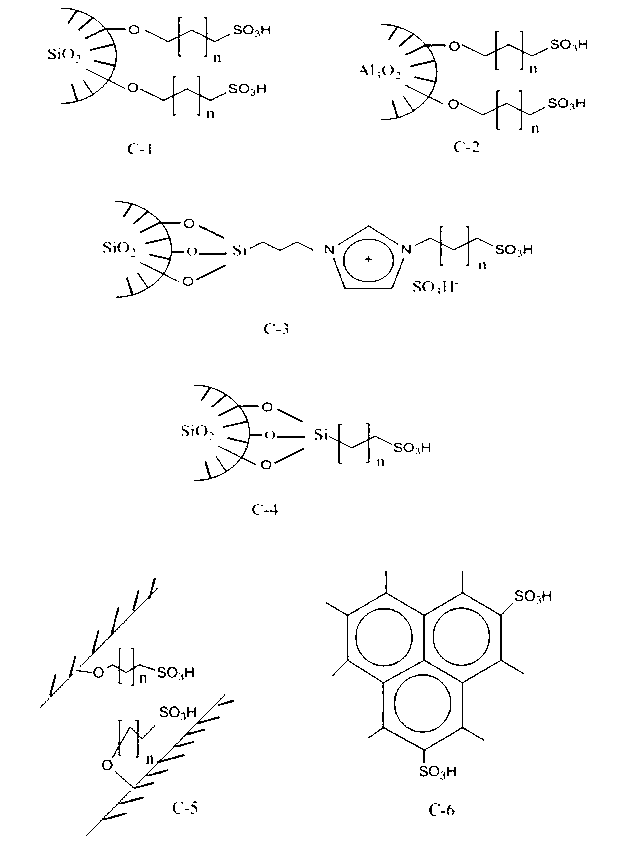
[0024] (1) Put 1,650g (95%) of pentaerythritol, 1,500g of cyclohexane, 2,550g of acrylic acid, and 55g of silica gel functionalized with sulfonic acid group into the reactor with reflux water separator, (the structure is as above, the catalyst of structural formula C-1 , n=1, its average pore diameter is 18.3nm, and its specific surface area is 480m 2 / g, sulfo group (SO 3 H) Functionalized density is 5gSO 3 H / 100g silica gel), p-hydroxyanisole 8g. Reflux dehydration esterification reaction for 10.8 hours, and 625 g of water was separated. Stop heating and reflux, and cool down to 50°C;
[0025] (2) Recover the C-1 catalyst by filtration. Under the situation of vigorous stirring, add quicklime to total 56g in the filtrate gradually, make material pH=6, then add magnesium polysilicate 70g, stir again 0.5 hour;
[0026] (3) Finally, decyclohexane was removed under reduced pressure at 60°C and suction filtered to obtain 3249 g of pentaerythritol triacrylate, a light yellow, ...
Embodiment 2
[0029] (1) Put 1650g (95%) of pentaerythritol, 1500g of benzene, 2550g of acrylic acid, and 127g of aluminum gel functionalized with sulfonic acid group into the reactor with reflux water separator (the structure is as above, the catalyst of structural formula C-2, n= 3. The average pore diameter is 14.2nm and the specific surface area is 210m 2 / g, sulfo group (SO 3 H) The functionalized density is 15gSO 3 H / 100g aluminum glue), hydroquinone 2.6g. Reflux dehydration esterification reaction for 7.5 hours, and 621 g of water was separated. Stop heating and reflux, and cool down to 50°C.
[0030] (2) Recover the C-2 catalyst by filtration. In the case of strong stirring, gradually add a total of 87g of alkaline calcium-based bentonite to the filtrate to make the material pH=6, then add 60g of activated carbon, and stir for another 0.5 hours.
[0031] (3) Finally, debenzene was removed under reduced pressure at 60°C and suction filtered to obtain 3228 g of pentaerythritol tr...
Embodiment 3
[0034] (1) Put 1650g (95%) of pentaerythritol (95%), 1200g of isopropyl ether, 2550g of acrylic acid, and 250g of silica gel functionalized with sulfonic acid group into the reactor with reflux water separator (the structure is as above, the catalyst of structural formula C-3, n =2, the average pore diameter is 10.5nm, and the specific surface area is 360m 2 / g, sulfo group (SO 3 H) The functionalized density is 30gSO 3 H / 100g silica gel), phenothiazine 32.3g, copper sulfate 4g. Reflux dehydration and esterification for 4.5 hours, and 630 g of water was separated. Stop heating and reflux, and cool down to 50°C.
[0035] (2) Recover the C-3 catalyst by filtration. Under the condition of vigorous stirring, gradually add 27g of sodium bicarbonate in total to the filtrate to make the material pH=6~7, then add 95g of alkaline calcium-based bentonite, and stir for another 0.5 hour.
[0036] (3) Finally, deisopropyl ether was removed under reduced pressure at 60°C, and suction fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com