Quorum-quenching enzyme OLB-26, and coding gene and application thereof
A quorum sensing and genetic technology, applied in the direction of transferase, hydrolase, microbial-based methods, etc., to achieve good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
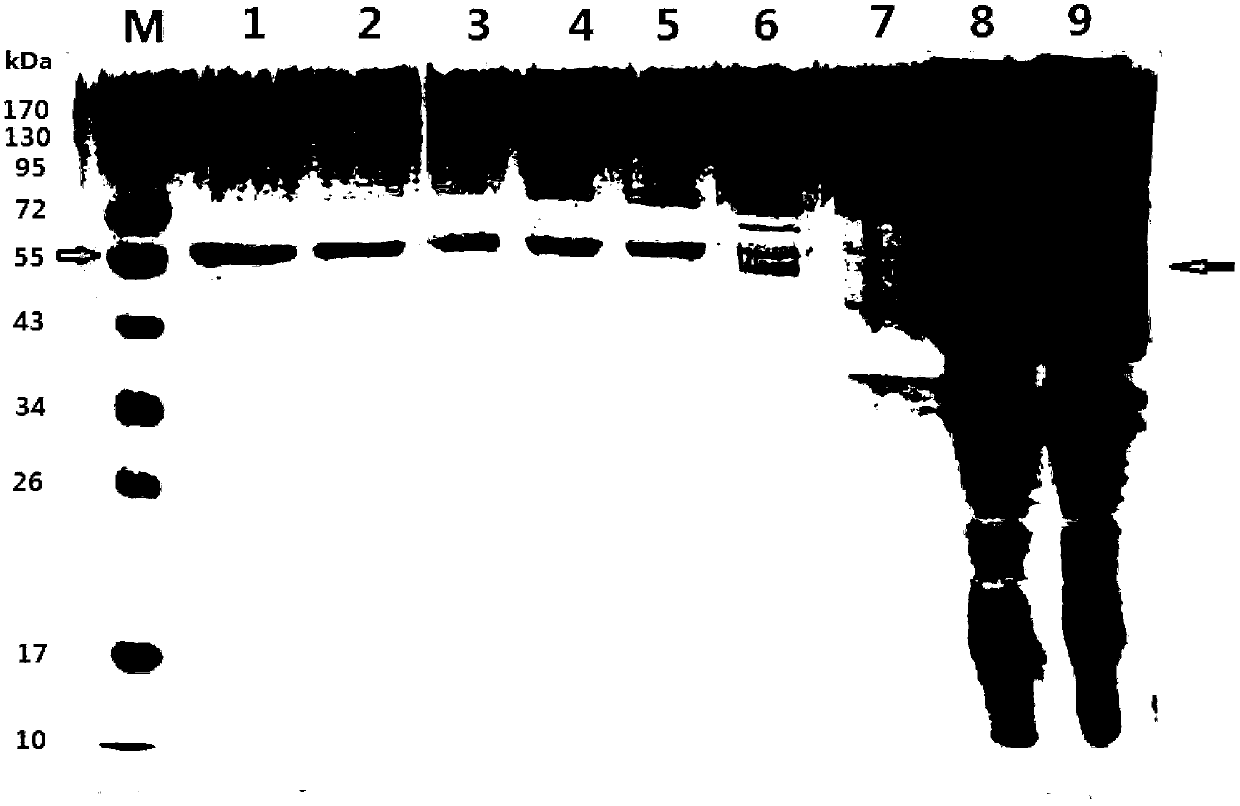
[0060] Embodiment 1, the preparation of fusion protein OLB-26
[0061] 1. Preparation of fusion gene olb-26
[0062] 1. Preparation of AiiO-AIO6 quencher gene fragment
[0063] ① artificially synthesized quencher enzyme gene aiiO-AIO6 shown in the 7th to 810th nucleotides from the 5' end of the sequence 2 of the sequence listing.
[0064] ②Using the gene synthesized in step ① as a template, perform PCR amplification with a primer pair consisting of A6-F (underlined EcoRI digestion sequence) and L2-Raiia-AIO6 (character shading marked Link sequence) to obtain a PCR product.
[0065] A6-F: 5′-CG GAATTC AAATCCCATGAAATCGAGACCAGTC-3′;
[0066] L2-Raiia-AIO6: 5'-GCTACCGCCGCCACCGGCCGTGCAGTCGCGCATGAAA-3'.
[0067] The enzyme used for PCR amplification was LA Taq (Takara, Japan). PCR amplification conditions: 95°C for 5min; 30 cycles of 94°C for 30s, 60°C for 30s, 72°C for 1min; 72°C for 10min.
[0068] ④Recover the PCR product.
[0069] 2. Preparation of AiiA-AI96 quencher gen...
Embodiment 2
[0114] Example 2. Characterization of the fusion protein OLB-26 as a quorum sensing quenching enzyme
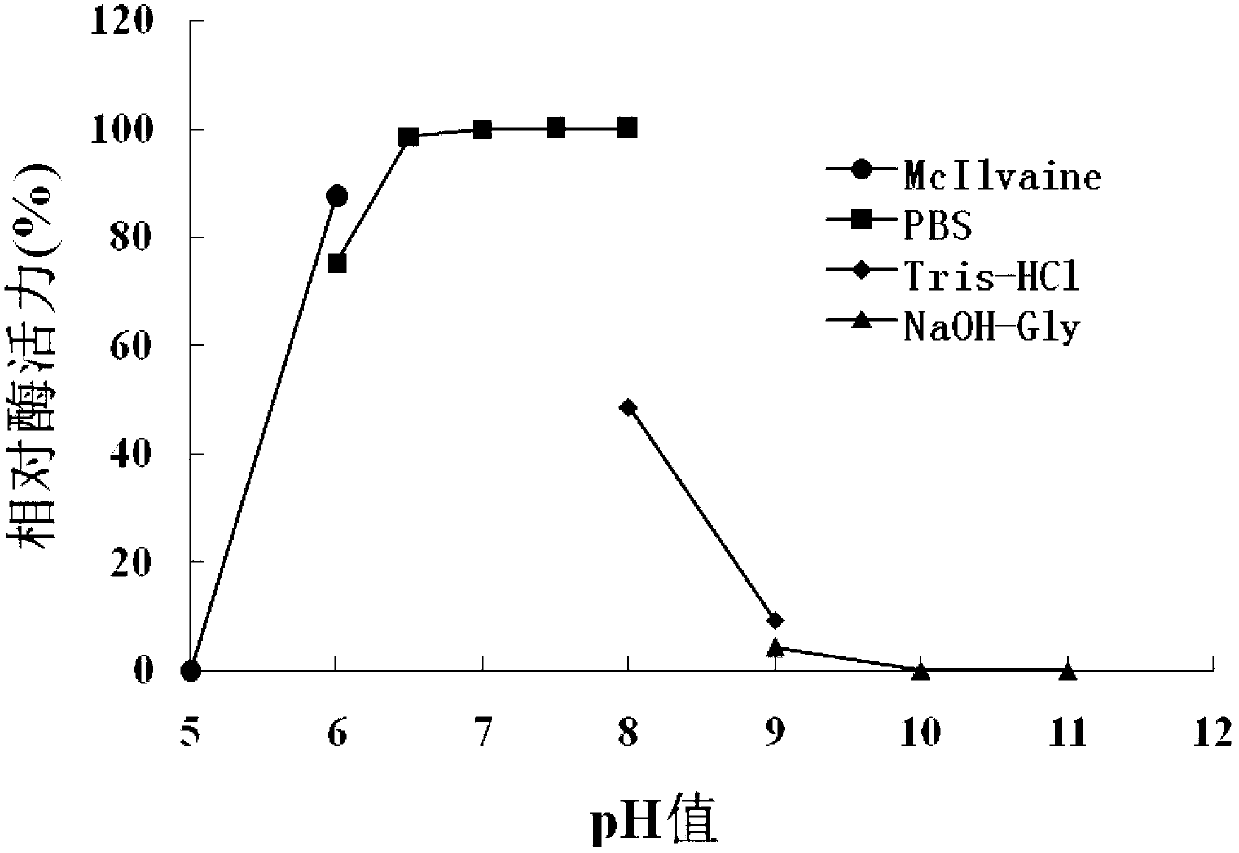
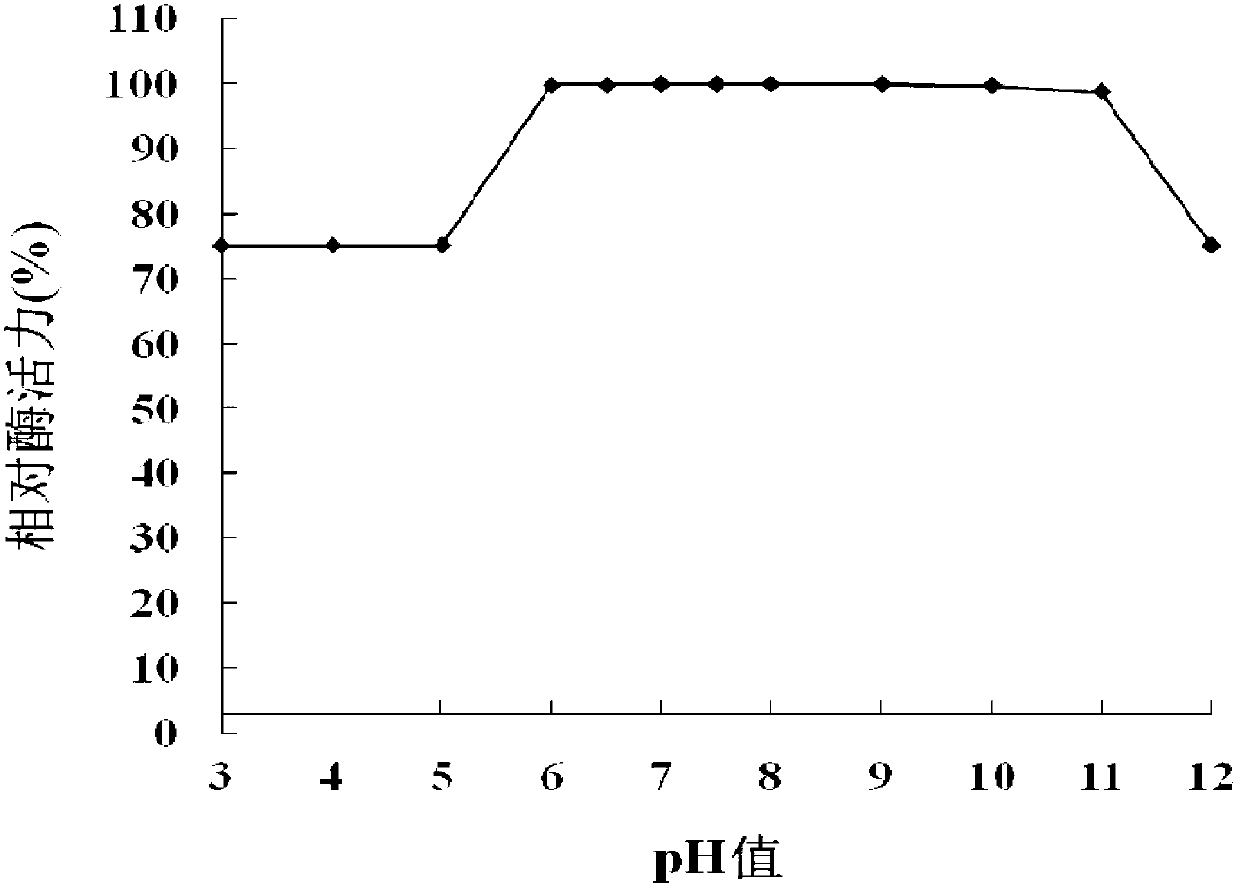
[0115] 1. Optimal pH
[0116] The purified OLB-26 protein solution (protein concentration: 0.49 mg / mL) prepared in Step 4 of Example 1 was used as the solution to be tested, and the quenching enzyme activity was detected at 35°C under different pH conditions to determine its maximum Appropriate pH.
[0117] The signal molecule in enzyme activity determination is 3-oxo-C8-HSL, and the following buffers are used respectively:
[0118] pH5.0, 0.1mol / L McIlvaine buffer (McIlvaine);
[0119] pH6.0, 0.1mol / L McIlvaine buffer;
[0120] pH6.0, 0.1mol / L PBS buffer (PBS);
[0121] pH6.5, 0.1mol / L PBS buffer;
[0122] pH7.0, 0.1mol / L PBS buffer;
[0123] pH7.5, 0.1mol / L PBS buffer;
[0124] pH8.0, 0.1mol / L PBS buffer;
[0125] pH8.0, 0.1mol / L Tris-HCl buffer (Tris-HCl);
[0126] pH9.0, 0.1mol / L Tris-HCl buffer;
[0127] pH9.0, 0.1mol / L glycine-NaOH buffer solution (NaOH-Gly); ...
Embodiment 3
[0180] Embodiment 3, fusion protein OLB-26 as the kinetic constant determination of quenching enzyme
[0181] 1. Use the purified OLB-26 protein solution (protein concentration: 0.49 mg / mL) prepared in Step 4 of Example 1 as the solution to be tested, and detect the enzyme activity of the quenching enzyme. The buffer solution is pH 8.0, 0.1 mol / mL L of PBS buffer, the reaction temperature is 35 ° C, the signal molecule is 3-oxo-C8-HSL, the final concentration in the reaction system is 18 μmol / L, in the reaction 1, 2, 3, 5, 10, 15, 20 , Stop the enzymatic reaction at 30min.
[0182] By calculating the ratio of enzyme activity to reaction time, if the ratio of the enzyme does not change within a certain period of time, the enzymatic reaction within this period of time is a first-order reaction to determine the K m and V max optimal response time.
[0183] Based on the determined first order reaction time, the K of the fusion protein OLB-26 was determined m value and V max T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com