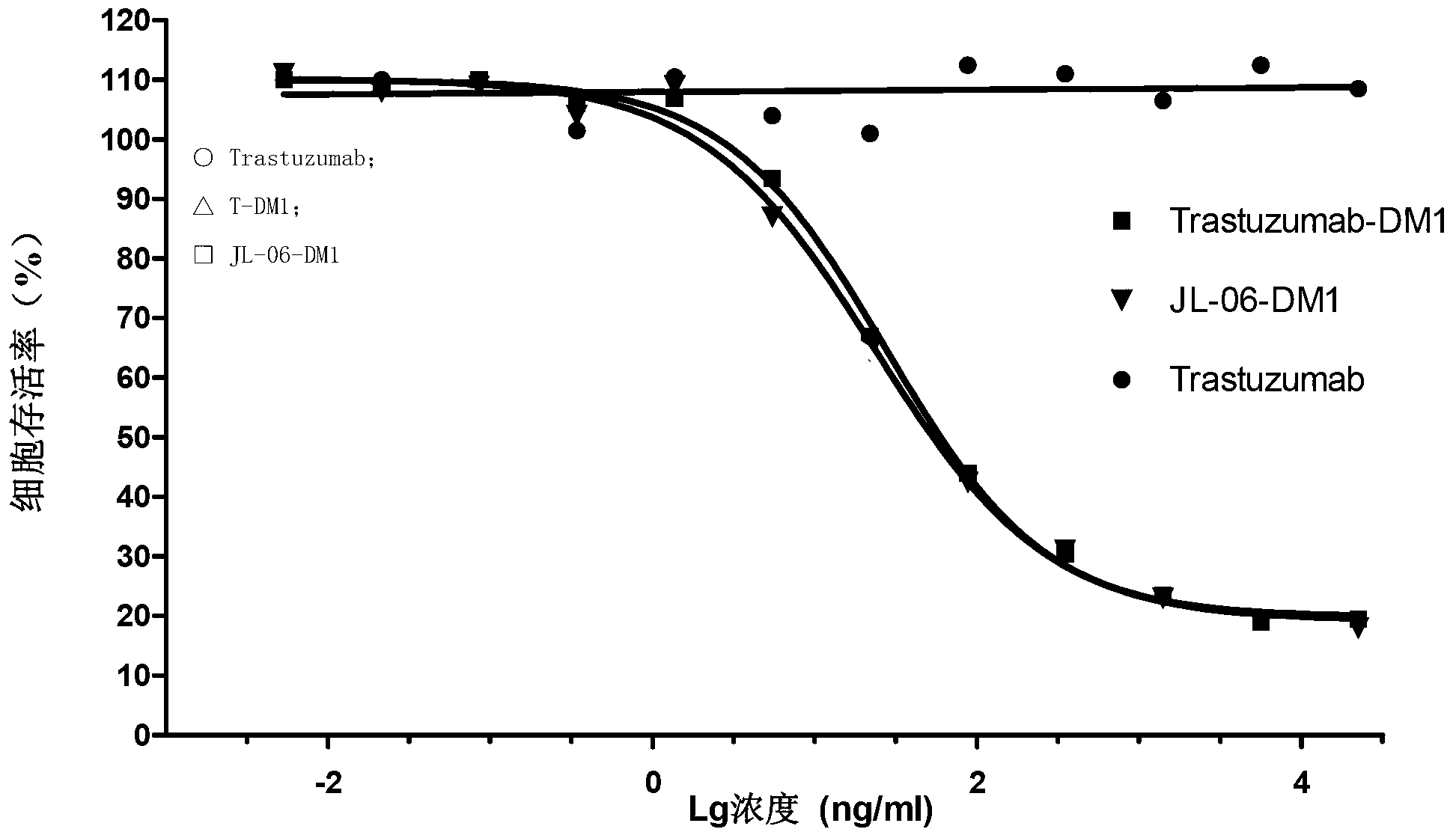
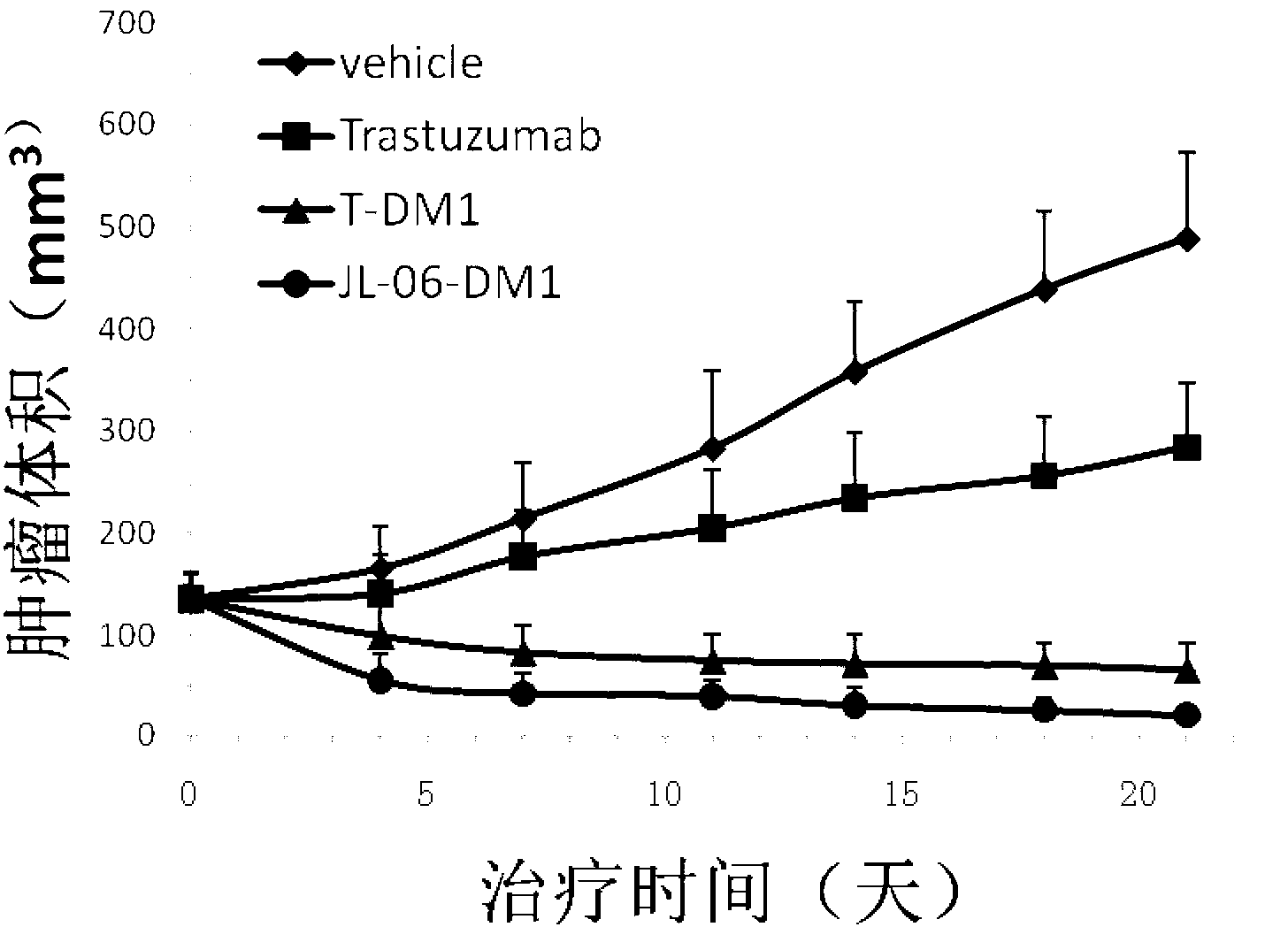
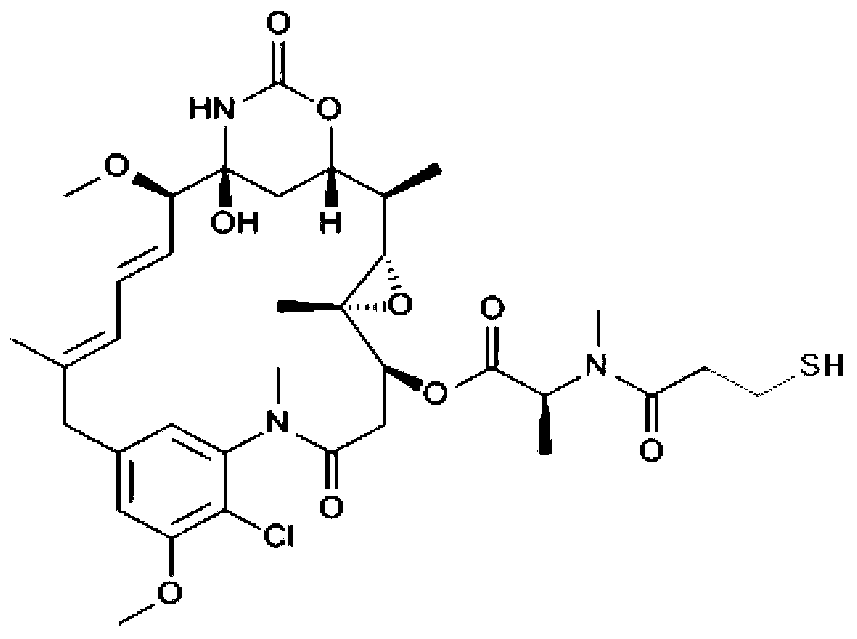
Anti-human ErbB2 antibody-ansamitocin conjugate and applications thereof
An antibody, trastuzumab technology, applied in the field of biomedicine, can solve the problems of decreased stability, increased toxicity, increased polymer components, etc., and achieves the effect of improving homogeneity and eliminating influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Construction and expression of anti-Her2 antibody Trastuzumab
[0045] DNA fragments encoding the heavy and light chains of Trastuzumab were synthesized from the whole gene and cloned into the antibody heavy and light chain expression vector PL101 respectively (Liu Yanjun et al., Chinese patent CN200610117245.8, the invention name is "anti-Her2 / ErbB2 antigen monoclonal antibody and its preparation Methods and Pharmaceutical Compositions", the announcement number is CN101165068B), the operations of enzyme cleavage and ligation were carried out according to the instructions of the kit provided by the business.
[0046] Trastuzumab antibody heavy chain amino acid sequence 1-449 is shown in SEQ ID NO: 1;
[0047] Trastuzumab antibody heavy chain amino acid sequence 1-214 is shown in SEQ ID NO:2.
[0048] The Trastuzumab heavy chain and light chain expression vectors constructed above were transformed into Escherichia coli DH5α, and positive clones were picked an...
Embodiment 2
[0051] Example 2: Trastuzumab mutant heavy chain K30R (JL-01)
[0052] Site-directed mutagenesis was performed on the DNA fragment encoding the heavy chain of Trastuzumab synthesized by conventional molecular biology techniques, and the heavy chain K30R of the Trastuzumab mutant was cloned into the expression vector of the antibody heavy chain, and the operation of enzyme digestion and ligation was carried out according to the instructions of the commercially provided kit. .
[0053] Trastuzumab mutant heavy chain K30R (JL-01) amino acid sequence 1-449 is shown in SEQ ID NO: 3;
[0054] The above constructed Trastuzumab mutant heavy chain K30R expression vector was transformed into Escherichia coli DH5α, and positive clones were picked and inoculated in 500ml LB medium for amplification. DNA was extracted and purified using Qiagen's Ultrapure Plasmid DNA Purification Kit (Ultrapure Plasmid DNA Purification Kit) according to the manufacturer's instructions. The above-mentione...
Embodiment 3
[0056] Example 3: Trastuzumab mutant heavy chain K65D (JL-02)
[0057] Using conventional molecular biology techniques, site-directed mutagenesis was performed on the DNA fragment encoding the heavy chain of Trastuzumab synthesized from the whole gene, and the heavy chain K65D of Trastuzumab mutant was cloned into the expression vector of the antibody heavy chain, and the operations of enzyme digestion and ligation were carried out according to the instructions of the commercially provided kit. .
[0058] Trastuzumab mutant heavy chain K65D (JL-02) amino acid sequence 1-449 is shown in SEQ ID NO:4;
[0059] The above constructed Trastuzumab mutant heavy chain K65D expression vector was transformed into Escherichia coli DH5α, and positive clones were picked and inoculated in 500ml LB medium for amplification. DNA was extracted and purified using Qiagen's Ultrapure Plasmid DNA Purification Kit (Ultrapure Plasmid DNA Purification Kit) according to the manufacturer's instructions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com