Synthetic method of neohesperidin dihydrochalcone
A technology for neohesperidin dihydrochalcone and hesperidin chalcone, which is applied in the field of synthesis of neohesperidin dihydrochalcone, can solve the problems of large amount of solvent, potential safety hazards, low content and the like, and achieves The effect of no catalyst, mild conditions and abundant water resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
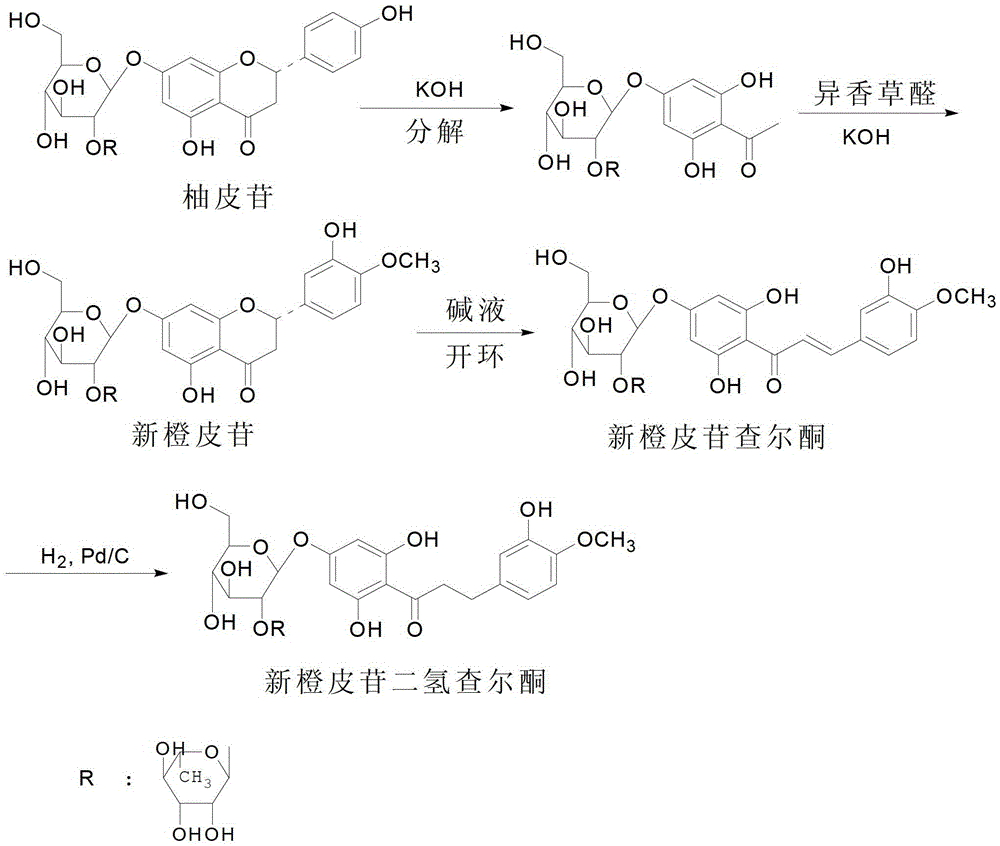
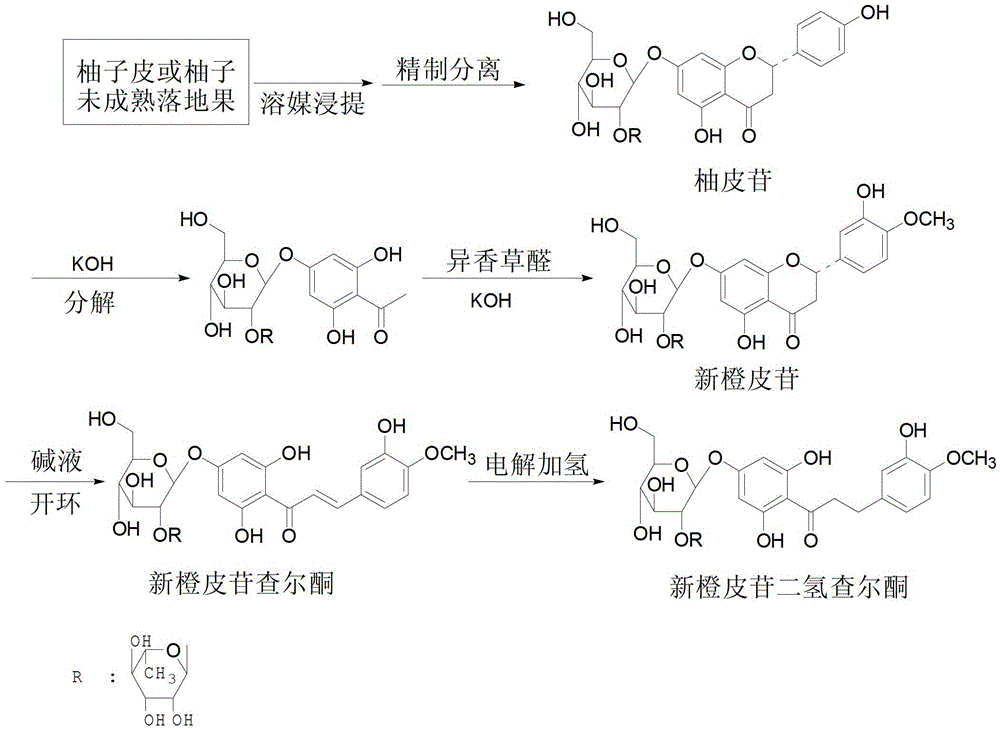
[0029] A kind of embodiment of the synthesis method of neohesperidin dihydrochalcone of the present invention, the process roadmap of described method is as attached figure 2 As shown, the method includes the following steps:
[0030] (1) Crush the fresh non-mildewed pomelo peel or immature pomelo fruit, then soak the crushed pomelo peel or immature pomelo fruit in ethanol with a purity of 80%, soak at a constant temperature of 40°C for 24 hours, and filter to separate The pomelo peel residue was used to obtain the naringin extract, the filtrate was collected, and the solvent was distilled off under reduced pressure with a rotary evaporator to obtain an extract;
[0031] (2) Separating and purifying the extract obtained in step (1) by column chromatography to obtain naringin; during column chromatography, polyamide is used as filler, and distilled water and 95% ethanol are used as eluent;
[0032] (3) Dissolving sodium hydroxide or potassium hydroxide in water to prepare an ...
Embodiment 2
[0039] A kind of embodiment of the synthetic method of neohesperidin dihydrochalcone of the present invention, described method comprises the following steps:
[0040] (1) Crush the fresh non-mildewed pomelo peel or immature pomelo fruit, then soak the crushed pomelo peel or immature pomelo fruit in ethanol with a purity of 80%, soak at a constant temperature of 40°C for 24 hours, and filter to separate The pomelo peel residue was used to obtain the naringin extract, the filtrate was collected, and the solvent was distilled off under reduced pressure with a rotary evaporator to obtain an extract;
[0041] (2) Separating and purifying the extract obtained in step (1) by column chromatography to obtain naringin; during column chromatography, polyamide is used as filler, and distilled water and 95% ethanol are used as eluent;
[0042] (3) Dissolving sodium hydroxide or potassium hydroxide in water to prepare an alkaline solution with a concentration of 5% by mass; then adding nar...
Embodiment 3
[0050] A kind of embodiment of the synthetic method of neohesperidin dihydrochalcone of the present invention, described method comprises the following steps:
[0051] (1) Crush the fresh non-mildewed pomelo peel or immature pomelo fruit, then soak the crushed pomelo peel or immature pomelo fruit in ethanol with a purity of 80%, soak at a constant temperature of 40°C for 24 hours, and filter to separate The pomelo peel residue was used to obtain the naringin extract, the filtrate was collected, and the solvent was distilled off under reduced pressure with a rotary evaporator to obtain an extract;
[0052] (2) Separating and purifying the extract obtained in step (1) by column chromatography to obtain naringin; during column chromatography, polyamide is used as filler, and distilled water and 95% ethanol are used as eluent;
[0053] (3) Dissolving sodium hydroxide or potassium hydroxide in water to prepare an alkaline solution with a concentration of 5% by mass; then adding nar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com