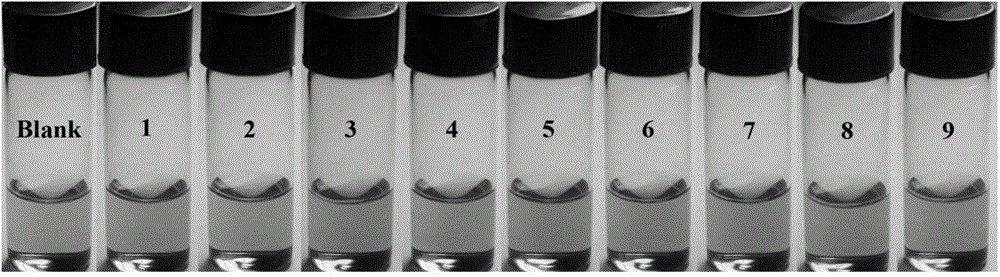
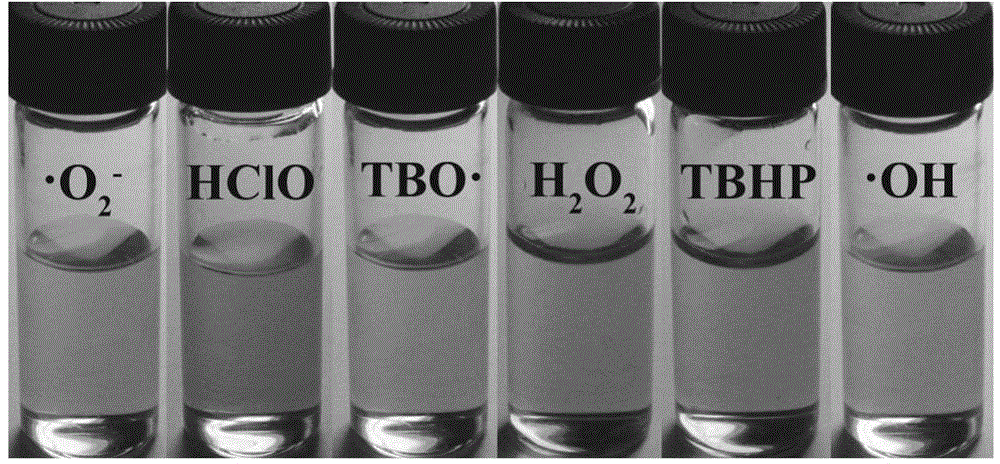
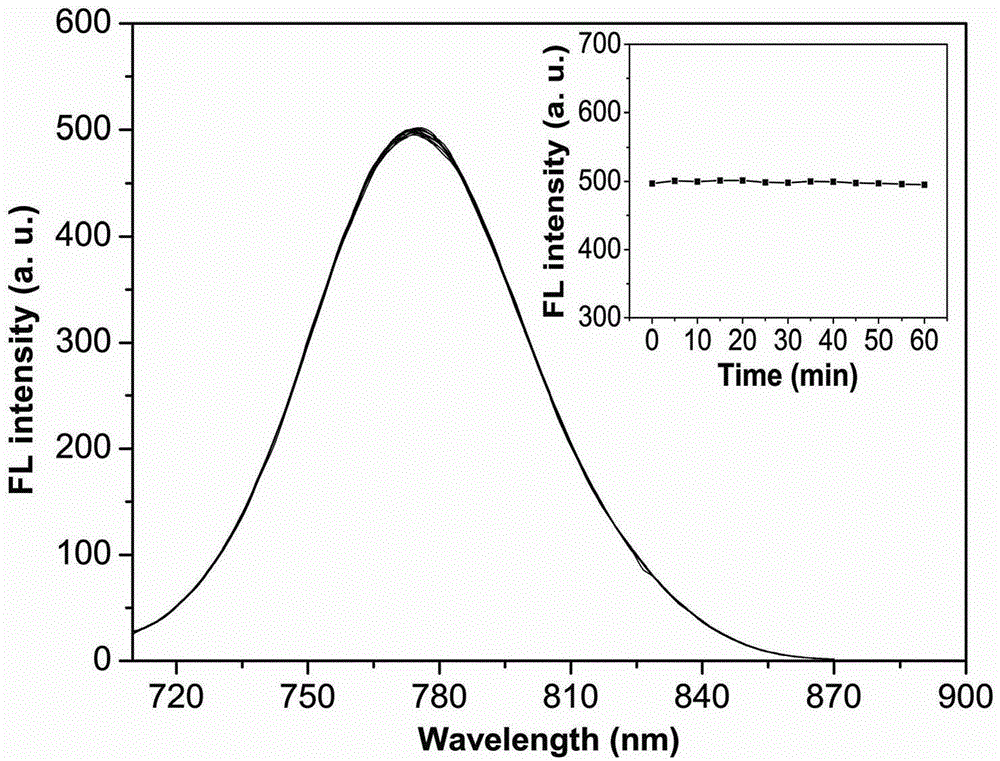
A dual-functional near-infrared fluorescent molecular probe for detecting hypochlorous acid and its preparation method
A fluorescent molecular probe, a technology for detecting hypochlorous acid, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of photoinstability, photobleaching performance, etc., achieve omission of pretreatment process, good selectivity , significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: fluorescent probe CyN(CH 2 R 1 )(CH 2 R 2 ) (R 1 =2-furyl, R 2 =2-pyridyl) preparation
[0047] Add 6ml of ethyl acetate to a 25ml single-necked round-bottomed flask, then add 2-aminopicoline (270mg, 2.5mmol) and furfural (200mg, 2.0mmol) in turn, and react at room temperature to obtain a brown solution, continue at room temperature Stir for 10 hours, remove the solvent ethyl acetate by rotary evaporation to obtain a brown substance which is the intermediate, add 5mL of anhydrous methanol to the obtained intermediate to dissolve, add sodium borohydride (150mg, 4.0mmol) under ice-water bath conditions, room temperature 2.5 hours under stirring, then add 10mL of saturated brine to quench the reaction, the aqueous phase was extracted 3 times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed to obtain a brown oily crude product; 150 mg of the crude product was dissolved in In 10mL of ...
Embodiment 2
[0052] Embodiment 2: fluorescent probe CyN(CH 2 R 1 )(CH 2 R 2 ) (R 1 =2-furyl, R 2 = phenyl) for the preparation of
[0053] Add 6ml of ethyl acetate to a 25ml single-necked round-bottom flask, then add benzylamine (268mg, 2.5mmol) and furfural (200mg, 2.0mmol) successively, react at room temperature to obtain a brown solution, continue to stir at room temperature for 10 Hours, the solvent ethyl acetate was removed by rotary evaporation to obtain a brown substance which was an intermediate, and 5 mL of anhydrous methanol was added to the obtained intermediate to dissolve, and sodium borohydride (150 mg, 4.0 mmol) was added under ice-water bath conditions, and stirred at room temperature for 2.5 hour, then add 10mL of saturated brine to quench the reaction, the aqueous phase was extracted 3 times with dichloromethane, the organic phase was combined, dried over anhydrous sodium sulfate, and the solvent was removed to obtain a brown oily crude product; 150 mg of the crude p...
Embodiment 3
[0056] Embodiment 3: fluorescent probe CyN(CH 2 R 1 )(CH 2 R 2 ) (R 1 =5-methyl-2-furyl, R 2 =2-pyridyl) preparation
[0057] Add 6ml of ethyl acetate to a 25ml single-necked round-bottom flask, then add 2-aminopicoline (270mg, 2.5mmol) and 5-methylfurfural (220mg, 2.0mmol) in sequence, and react at room temperature to obtain a brown solution , continue to stir at room temperature for 10 hours, remove the solvent ethyl acetate by rotary evaporation to obtain a brown substance which is the intermediate, add 5 mL of anhydrous methanol to the obtained intermediate to dissolve, add sodium borohydride (150 mg, 4.0 mmol), stirred at room temperature for 2.5 hours, then added 10 mL of saturated brine to quench the reaction, the aqueous phase was extracted 3 times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed to obtain a brown oily crude product; 150mg of the crude product was dissolved in 10mL of anhydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com