Lithium iodide organic electrolyte for lithium iron battery and preparation method thereof
An organic electrolyte, lithium iodide technology, applied in the field of lithium batteries, can solve the problems of increased electrolyte viscosity, poor safety performance, scrapping, etc., to improve low-temperature discharge, meet safety and environmental protection requirements, and reduce costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
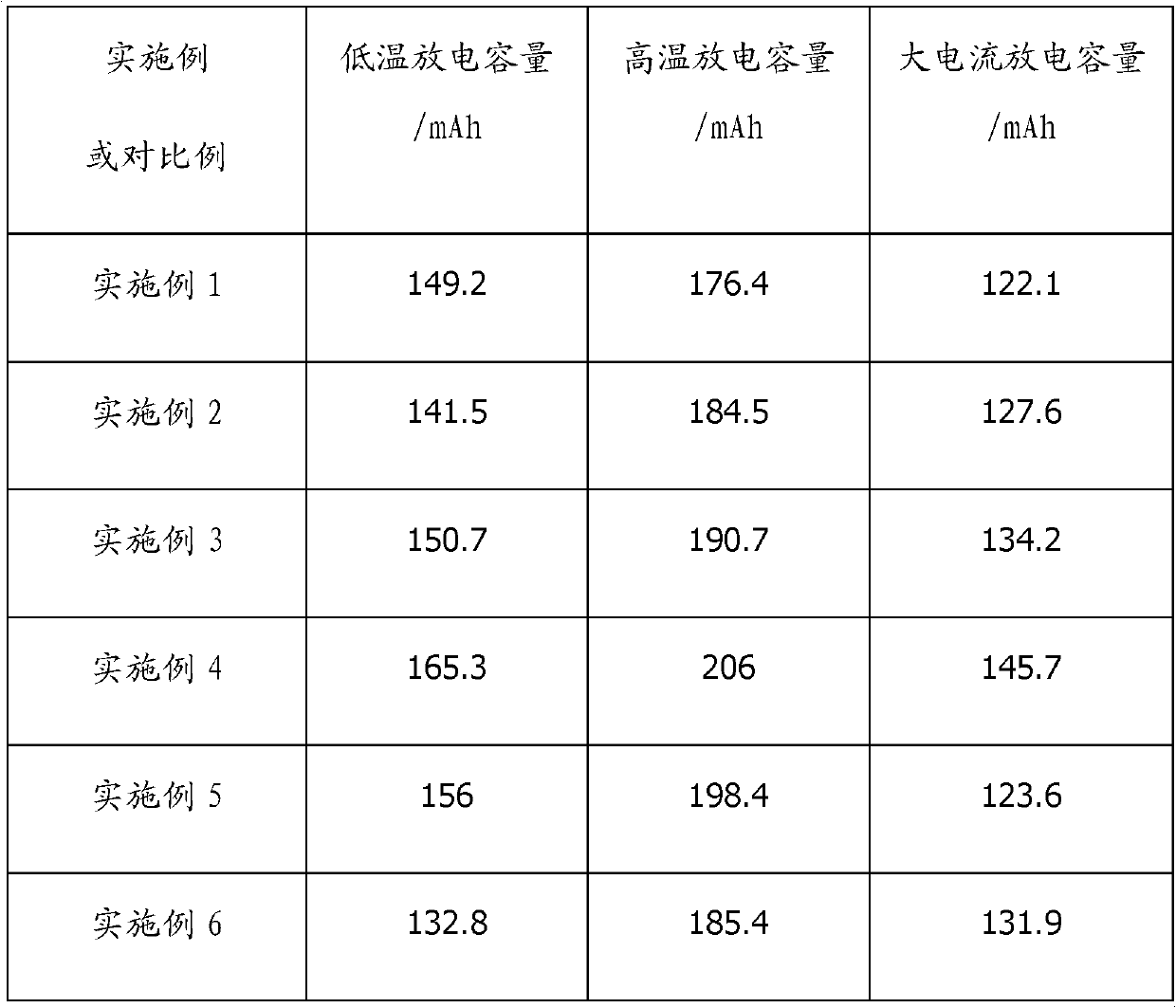
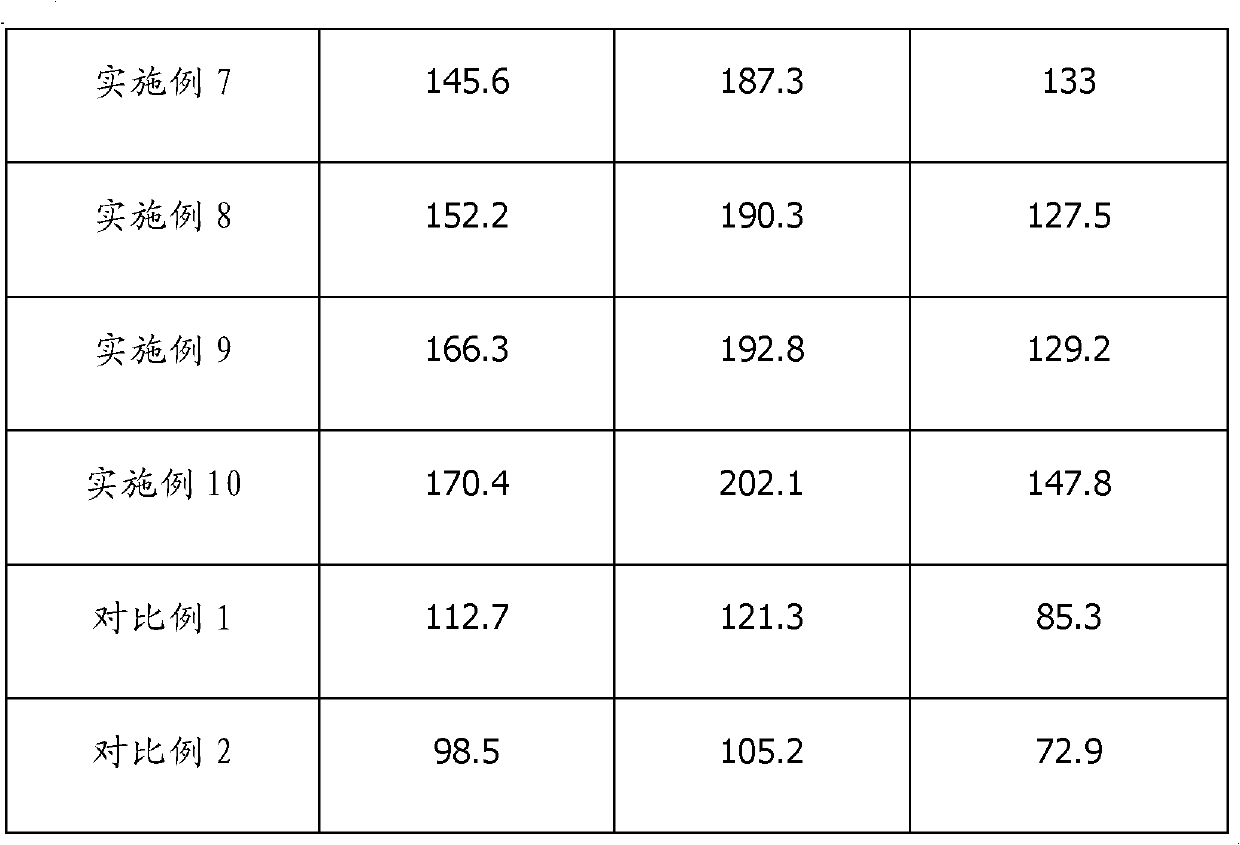
Examples
Embodiment 1
[0035] Mix 30% of the electrolyte mass fraction of ethylene glycol dimethyl ether, 30% of 1,3-dioxolane, and 25% of dimethyl sulfoxide, and then add the electrolyte mass fraction of 1.5% 2,6-di-tert-butyl-4-methylphenol and 3% 3-methylisoxazole, the above-mentioned raw materials are all dehydrated with ordinary molecular sieve before mixing; after mixing uniformly, add the electrolyte mass The fraction is 8% anhydrous lithium iodide and 2.5% lithium trifluoromethanesulfonate. After completely dissolving, use lithiated molecular sieve for dehydration and adsorption, adsorb for 48 hours, and filter to obtain lithium iodide organic electrolyte when the moisture reaches 60ppm .
Embodiment 2
[0037] Mix 1,3-dioxolane, 10% tetrahydrofuran, 20% sulfolane, and 25% vinyl sulfone that account for 30% of the electrolyte mass fraction, and add 0.5% of the electrolyte to the mixed solution. Butylhydroquinone and 1.5% 5-methylisoxazole, the above raw materials are all dehydrated with ordinary molecular sieve before mixing; after mixing uniformly, add anhydrous lithium iodide and 10% of the electrolyte mass fraction 3% lithium bis(trifluoromethylsulfonyl)imide is completely dissolved and then dehydrated and adsorbed using lithiated molecular sieve for 72 hours. When the moisture reaches 65 ppm, an organic lithium iodide electrolyte is obtained by filtration.
Embodiment 3
[0039] Mix ethylene glycol dimethyl ether, 20% sulfolane, and 30% dimethyl sulfoxide, which accounts for 30% of the electrolyte mass fraction, and add 2,6-di-tert, which accounts for 2% of the electrolyte mass fraction, into the mixed solution. Butyl-4-methylphenol and 1% 3,5-dimethyl-4-iodoisoxazole, the above-mentioned raw materials are all dehydrated with ordinary molecular sieve before mixing; after mixing uniformly, add the weight of electrolyte Anhydrous lithium iodide with a fraction of 17% is completely dissolved and then dehydrated and adsorbed using lithiated molecular sieve for 72 hours. When the moisture reaches 50 ppm, an organic lithium iodide electrolyte is obtained by filtration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com