Cyclic biodegradation aliphatic polyester and preparation method thereof
An aliphatic polyester, biodegradable technology, applied in the production of bulk chemicals, etc., to achieve mild reaction conditions and mild specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Step (1): Add propynyl alcohol and L-lactide with a molar ratio of 1:40 into a specific microwave reaction tube after silanization, and add stannous octoate with 0.5% of the mass of L-lactide monomer, Repeatedly evacuate and pass N 2 After heating for about 1 hour to melt and mix the reaction system uniformly, place the reaction tube in a DISCOVER microwave reactor and polymerize under the assistance of microwaves. The reaction temperature is set at 100°C, the microwave power is 30W, and the reaction time is 20 minutes; After the reaction, the solid crude product was dissolved in chloroform, precipitated with absolute ethanol, and then dried in vacuum at 45°C to constant weight to obtain single-terminal alkynylated poly(L-lactide).
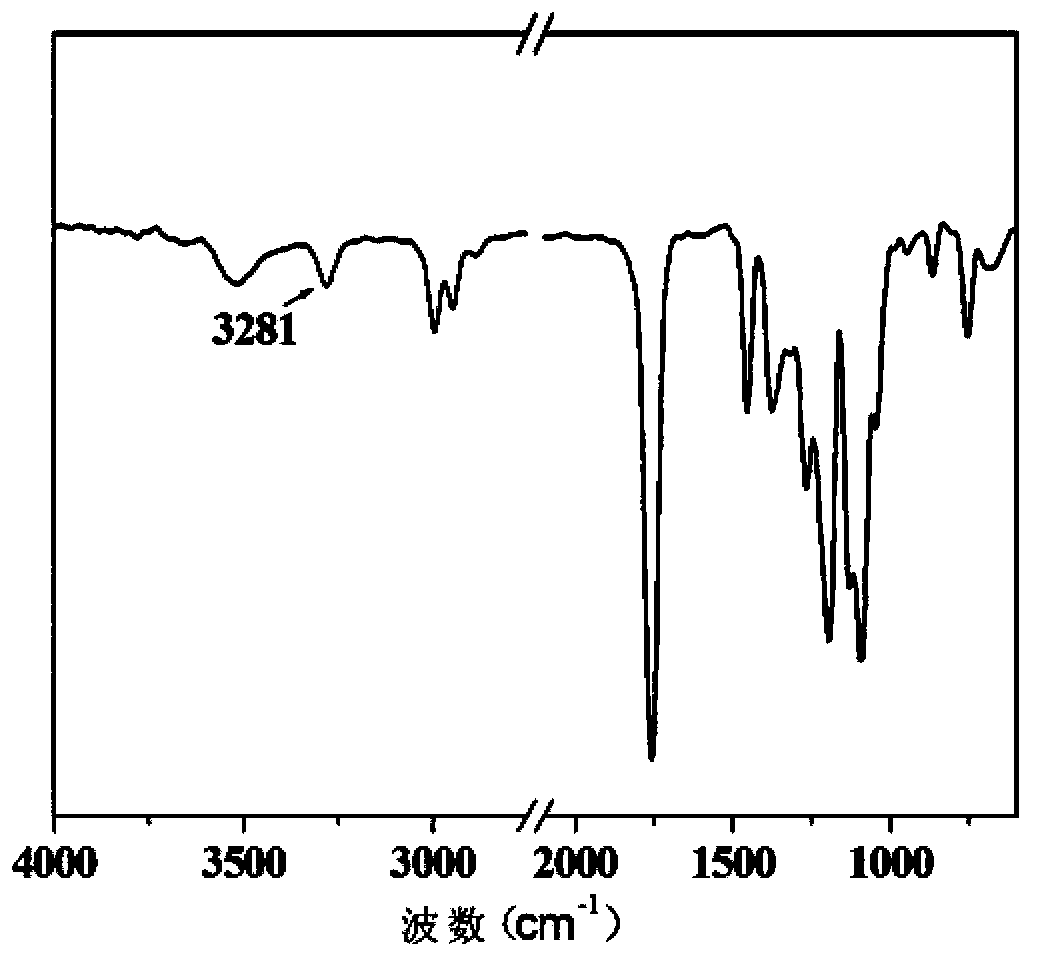
[0038] The composition and structure of the single-end alkynylated poly(L-lactide) synthesized in this example were characterized by infrared spectroscopy. figure 1 . It can be observed from the figure that the 1756cm -1 The characterist...
Embodiment 2
[0044] Step (1): Add propynyl alcohol and ε-caprolactone with a molar ratio of 1:40 into a specific microwave reaction tube after silanization, and add stannous octoate with 1% mass of ε-caprolactone monomer, Repeatedly evacuate and pass N 2 After heating for about 1 hour to melt and mix the reaction system uniformly, the reaction tube was placed in a DISCOVER microwave reactor and polymerized under the assistance of microwaves. The reaction temperature was set at 110°C, the reaction time was 15 minutes, and the microwave power was 50W. It was a transparent viscous liquid, then heated to 140°C, and continued to react for 20 minutes; after the reaction, the solid crude product was dissolved in chloroform, precipitated in absolute ethanol, and then dried in vacuum at 45°C to constant weight to obtain a of poly(ε-caprolactone).
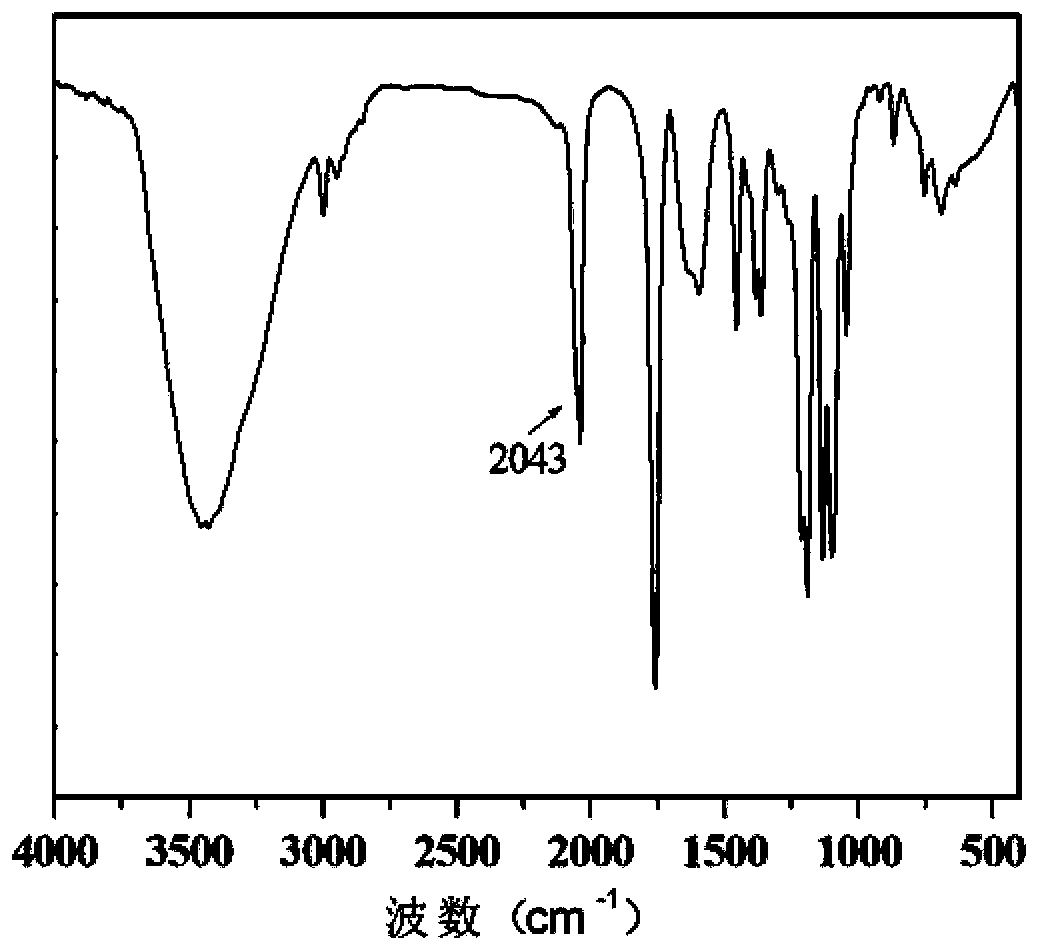
[0045] Step (2): Weigh 1 mmol of single-terminal alkynylated poly(ε-caprolactone) obtained in step (1), put it in a 100 mL three-neck flask, add 50 mL ...
Embodiment 3
[0050] Step (1): Add 3,5-dimethyl-1-hexyn-3-ol and δ-valerolactone with a molar ratio of 1:80 into a specific microwave reaction tube after silanization, and add δ- Tin lactate with 2% mass of valerolactone monomer, repeated vacuuming and N 2 After heating for about 1 hour to melt and mix the reaction system uniformly, the reaction tube was placed in a DISCOVER microwave reactor and polymerized under the assistance of microwaves. The reaction temperature was set at 90°C, the reaction time was 60 minutes, and the microwave power was 50W. It was a transparent viscous liquid, and immediately heated to 140°C, and continued to react for 30 minutes; the solid crude product was dissolved in chloroform, precipitated in absolute ethanol, and then vacuum-dried at 45°C for 24 hours, and then vacuum-dried at 45°C to constant weight. Single-ended alkynylated poly(δ-valerolactone) was obtained.
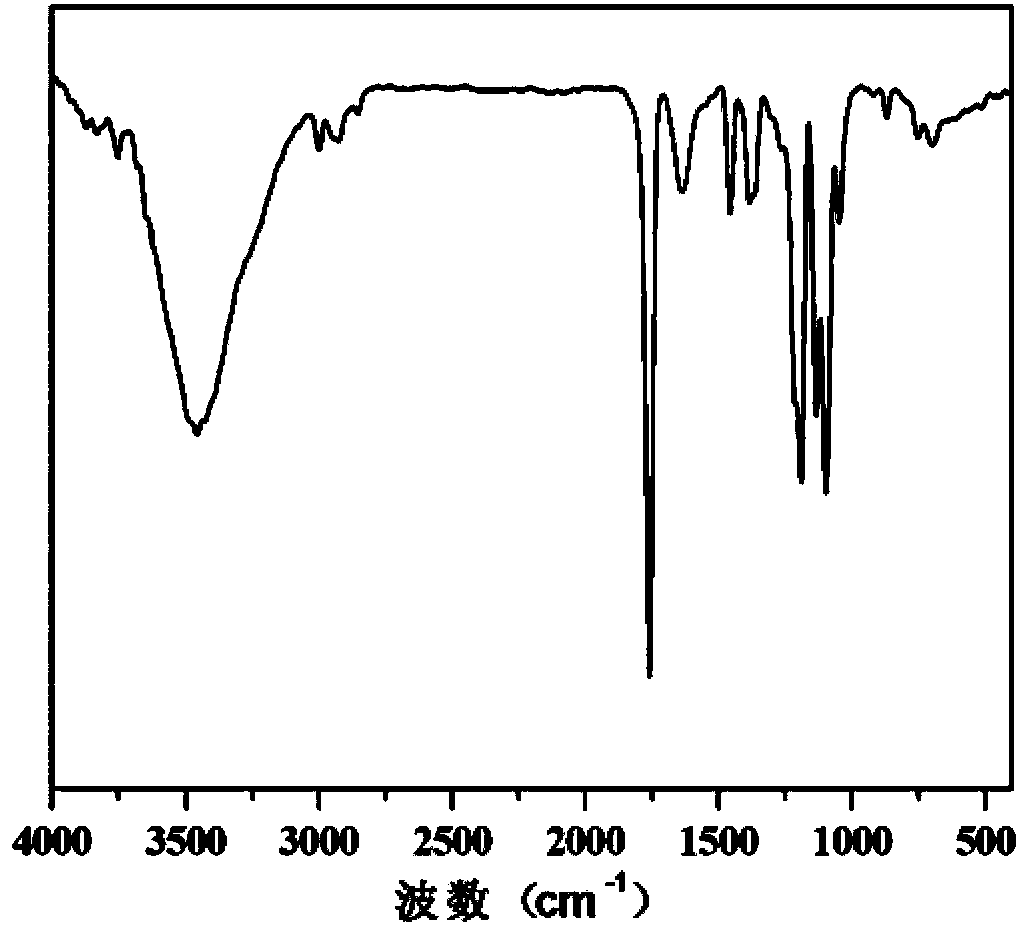
[0051] Step (2): Weigh 1 mmol of the poly(δ-valerolactone) modified by the terminal alkyne gro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com