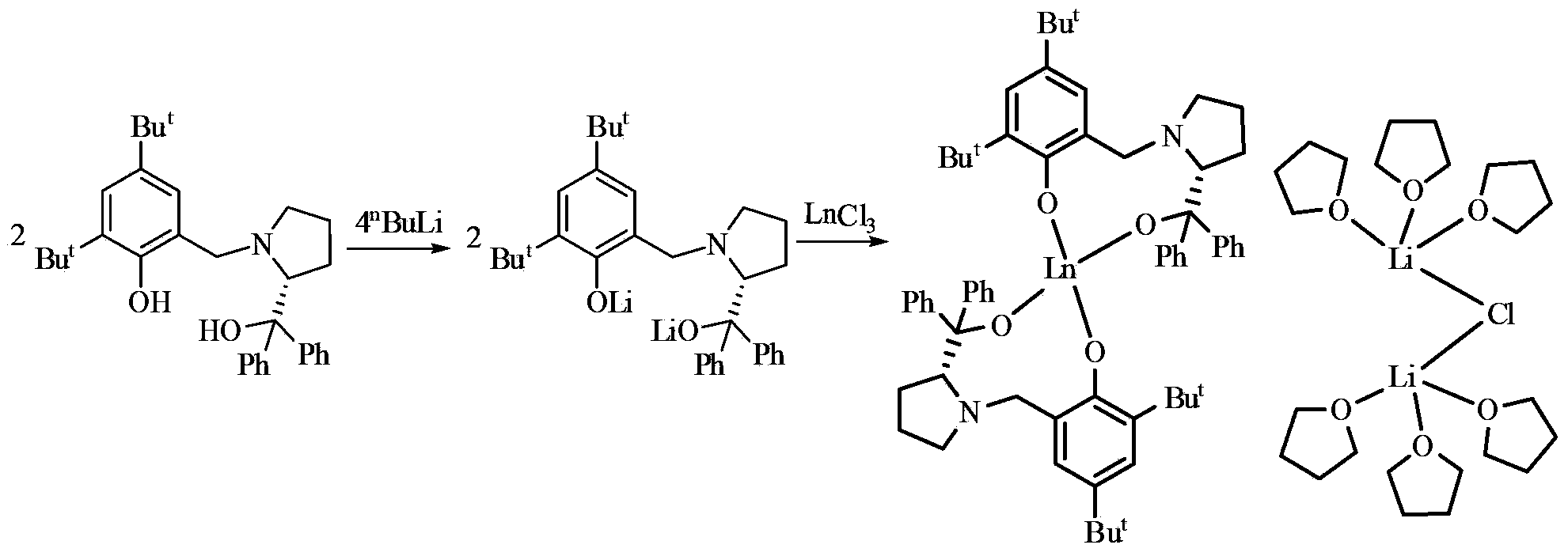
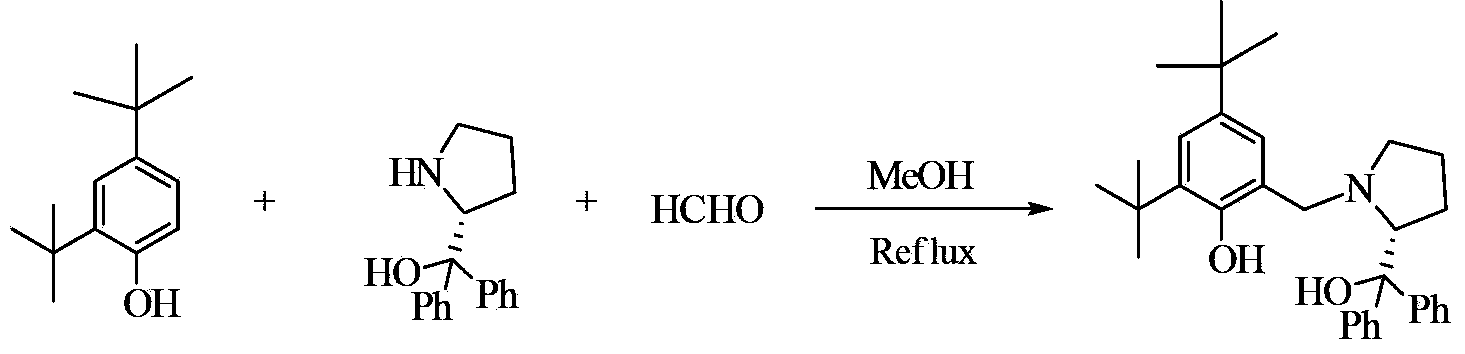Preparation method of chiral epoxy compound
A technology of epoxy compounds and rare earth compounds, applied in chemical instruments and methods, chemical/physical processes, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems that have not been seen yet, and reach the scope of substrate adaptation wide range, mild reaction conditions and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
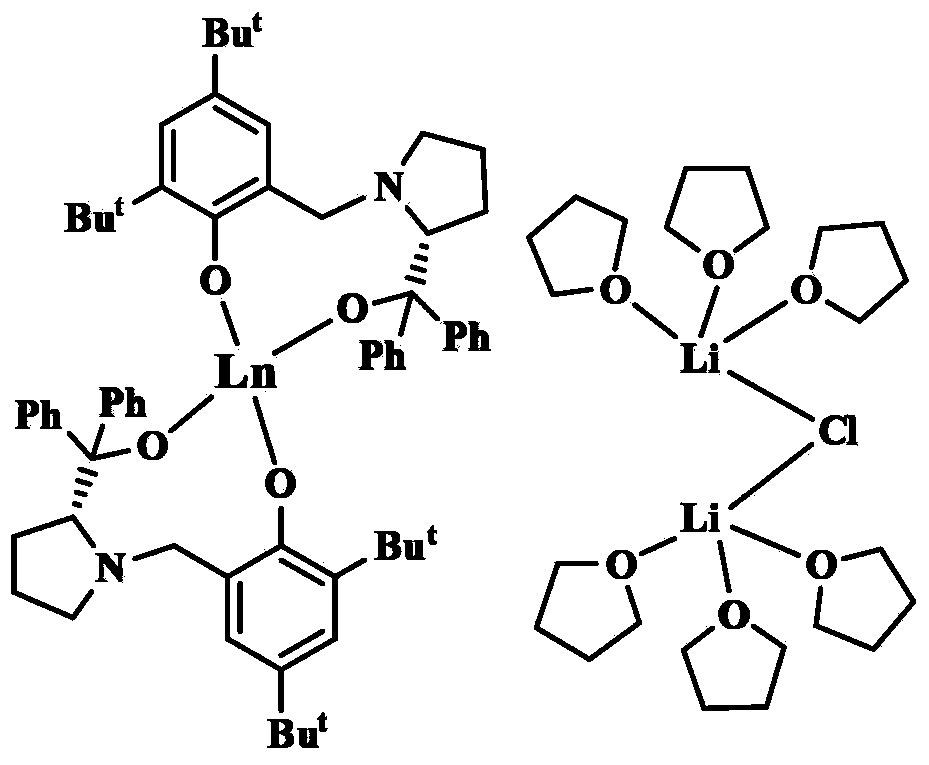
[0056] Embodiment one: adopt above-mentioned obtained [YL 2 ][{(THF) 3 Li} 2 (μ-Cl)] catalyzed asymmetric epoxidation of α,β-unsaturated ketones:
[0057] In the reaction flask that has been treated with dehydration and deoxygenation, add 0.0625 grams (0.3 mmol) of chalcone (that is, chalcone without substituents, the same as in Examples 2 to 5) and 0.048 grams (0.03 mmol) of catalyst under the protection of argon. mmol), add 2.0 ml of tetrahydrofuran, stir in a constant temperature bath with a set temperature of 25°C for 5 minutes, then add 0.065 ml of tert-butyl hydroperoxide (5.5 mmol / ml n-decane solution), and continue at 25°C After reacting for 4h, the reaction was terminated with saturated aqueous sodium sulfite solution.
[0058] The product was separated on a silica gel column and washed with an eluent of ethyl acetate:petroleum ether=1:30 to obtain 57.80 mg of epoxidized chalcone with a yield of 95%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralp...
Embodiment 2
[0059] Embodiment two: adopt the [YbL that above-mentioned makes 2 ][{(THF) 3 Li} 2 (μ-Cl)] catalyzed asymmetric epoxidation of α,β-unsaturated ketones:
[0060] In the reaction flask that has been treated with dehydration and deoxygenation, add 0.104 g (0.5 mmol) of chalcone and 0.076 g (0.05 mmol) of catalyst under the protection of argon, add 3.2 ml of tetrahydrofuran, and set the temperature at 0 ° C After stirring in a constant temperature bath for 20 minutes, 0.11 ml of tert-butyl hydroperoxide (5.5 mmol / ml n-decane solution) was added, and the reaction was continued at 0°C for 12 h, and terminated with saturated aqueous sodium sulfite.
[0061] The product was separated on a silica gel column and washed with an eluent of ethyl acetate:petroleum ether=1:30 to obtain 78.4 mg of epoxidized chalcone with a yield of 70%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak OJ column, eluent i-PrOH / hexane (10 / 90), flow rate 1.0mL / min, ee value 89%.
Embodiment 3
[0062] Embodiment three: adopt the [SmL that above-mentioned makes 2 ][{(THF) 3 Li} 2 (μ-Cl)] catalyzed asymmetric epoxidation of α,β-unsaturated ketones:
[0063] In the reaction flask that has been treated with dehydration and deoxygenation, add 0.0625 grams (0.3 mmol) of chalcone and 0.048 grams (0.03 mmol) of catalyst under the protection of argon, add 1.9 milliliters of tetrahydrofuran, and set the temperature at 25 ° C After stirring in a constant temperature bath for 5 minutes, 0.065 ml of tert-butyl hydroperoxide (5.5 mmol / ml n-decane solution) was added, and the reaction was continued at 25°C for 4 h, and terminated with saturated aqueous sodium sulfite.
[0064] The product was separated on a silica gel column and rinsed with an eluent of ethyl acetate:petroleum ether=1:30 to obtain 63.2 mg of epoxidized chalcone with a yield of 94%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak OJ column, eluent i-PrOH / hexane (10 / 90), flow rate 1.0mL / min, ee ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com