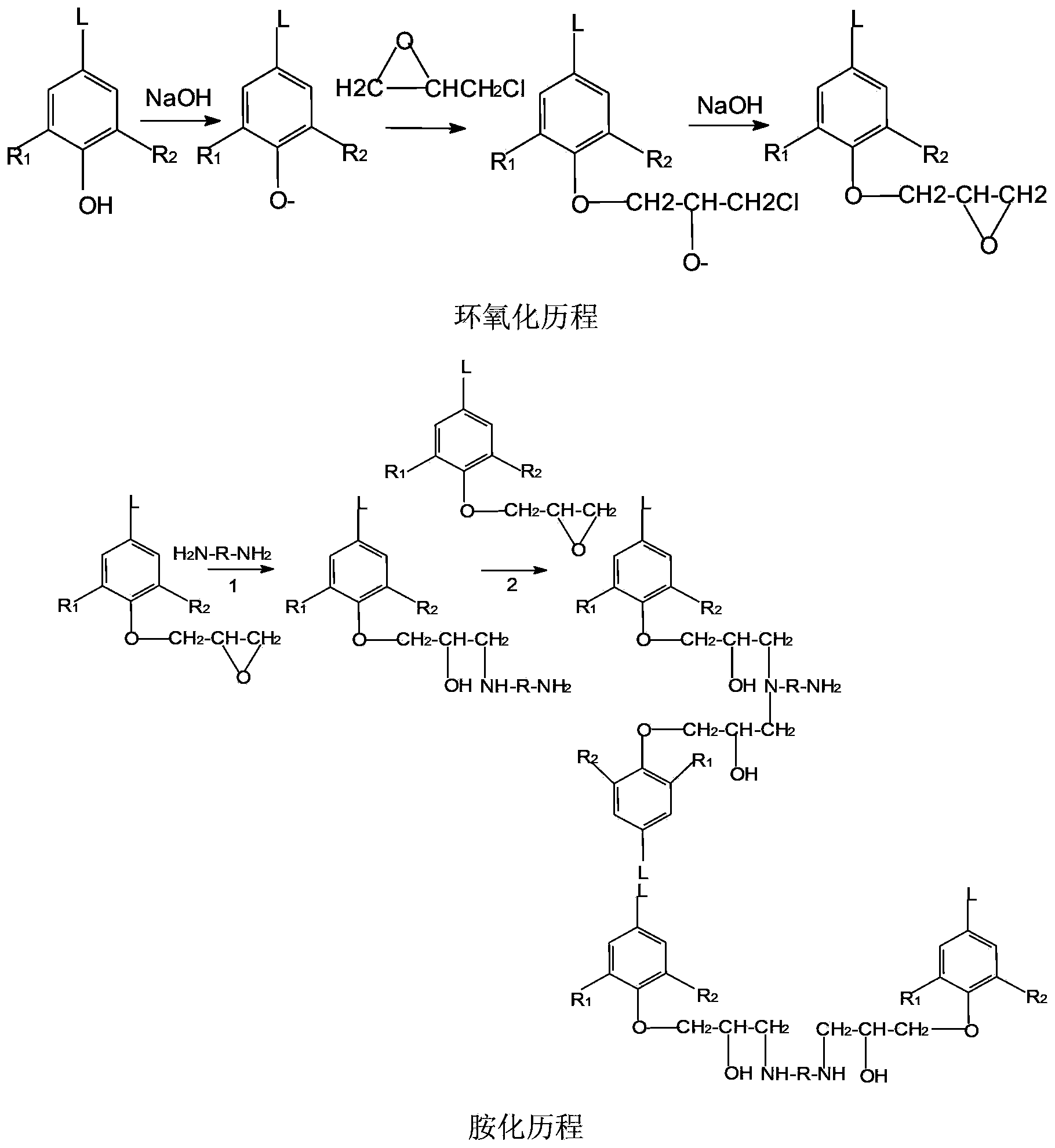
Amination lignin containing reactive amino and preparation and application thereof
A technology of aminated lignin and reactivity, which is applied in the field of lignin, can solve problems such as no related reports, and achieve the effects of cost reduction, simple preparation method, and improvement of reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
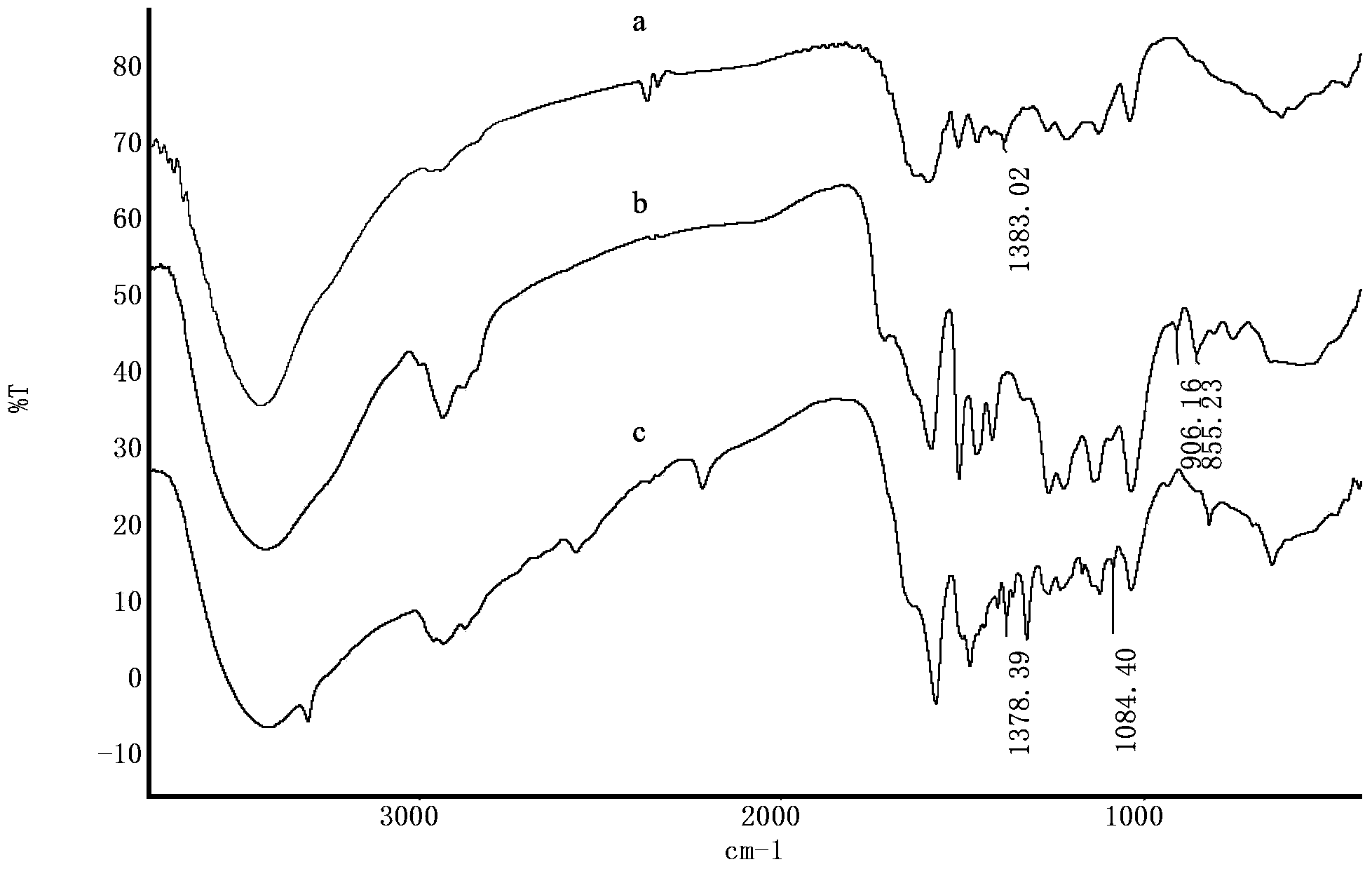
[0043] Four-neck bottle, with a thermometer inserted into two ports, a dropping funnel, a condenser tube in one port, a stopper in the other port, and magnetic stirring. Dissolve 5 grams of degraded and purified small molecule alkali lignin in 50 milliliters of deionized water, add 1.5 grams of sodium hydroxide into the three-necked bottle at the same time, stir to dissolve completely, and slowly add epichlorohydrin 2.5 g, while adding epichlorohydrin dropwise, 1.5 g of sodium hydroxide was added in batches, 4 times in total, keeping the pH of the reaction system greater than 12, and reacting for 8 hours. After the reaction, add water to wash several times to neutrality, centrifugal precipitation to remove alkali agent and unreacted lignin, add acetone to wash several times, centrifugal precipitation to remove unreacted epichlorohydrin. A black solid was obtained, dried in a vacuum oven at 40°C, and ground to obtain epoxy lignin. The test epoxy value is 0.2%, and it is ready ...
Embodiment 2
[0047] Four-neck bottle, with a thermometer inserted into two ports, a dropping funnel, a condenser tube in one port, a stopper in the other port, and magnetic stirring. Dissolve 5 grams of degraded and purified small molecule alkali lignin in 50 ml of deionized water, add 1.5 g of sodium hydroxide into the three-necked bottle at the same time, stir to dissolve completely, and slowly add 2.5 g of epoxy For chloropropane, add 1.5 g of sodium hydroxide in batches at the same time as adding epichlorohydrin dropwise, for a total of 4 times, keep the pH of the reaction system greater than 12, and react for 8 hours. After the reaction, add water to wash several times to neutrality, centrifugal precipitation to remove alkali agent and unreacted lignin, add acetone to wash several times, centrifugal precipitation to remove unreacted epichlorohydrin. A black solid was obtained, dried in a vacuum oven at 40°C, and ground to obtain epoxy lignin. Test epoxy value is 0.196%, stand-by.
...
Embodiment 3
[0051] Four-neck bottle, with a thermometer inserted into two ports, a dropping funnel, a condenser tube in one port, a stopper in the other port, and magnetic stirring. Dissolve 10 grams of degraded and purified small molecule alkali lignin in 50 ml of deionized water, add 3 g of sodium hydroxide into the three-necked bottle at the same time, stir to dissolve completely, and slowly add epichlorohydrin 5.5 g, while adding epichlorohydrin dropwise, 3 g of sodium hydroxide was added in batches for a total of 4 times, keeping the pH of the reaction system greater than 12, and reacting for 8 hours. After the reaction, add water to wash several times to neutrality, centrifugal precipitation to remove alkali agent and unreacted lignin, add acetone to wash several times, centrifugal precipitation to remove unreacted epichlorohydrin. A black solid was obtained, dried in a vacuum oven at 40°C, and ground to obtain epoxy lignin. Test epoxy value is 0.206%, stand-by.
[0052] 1.5 grams...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com