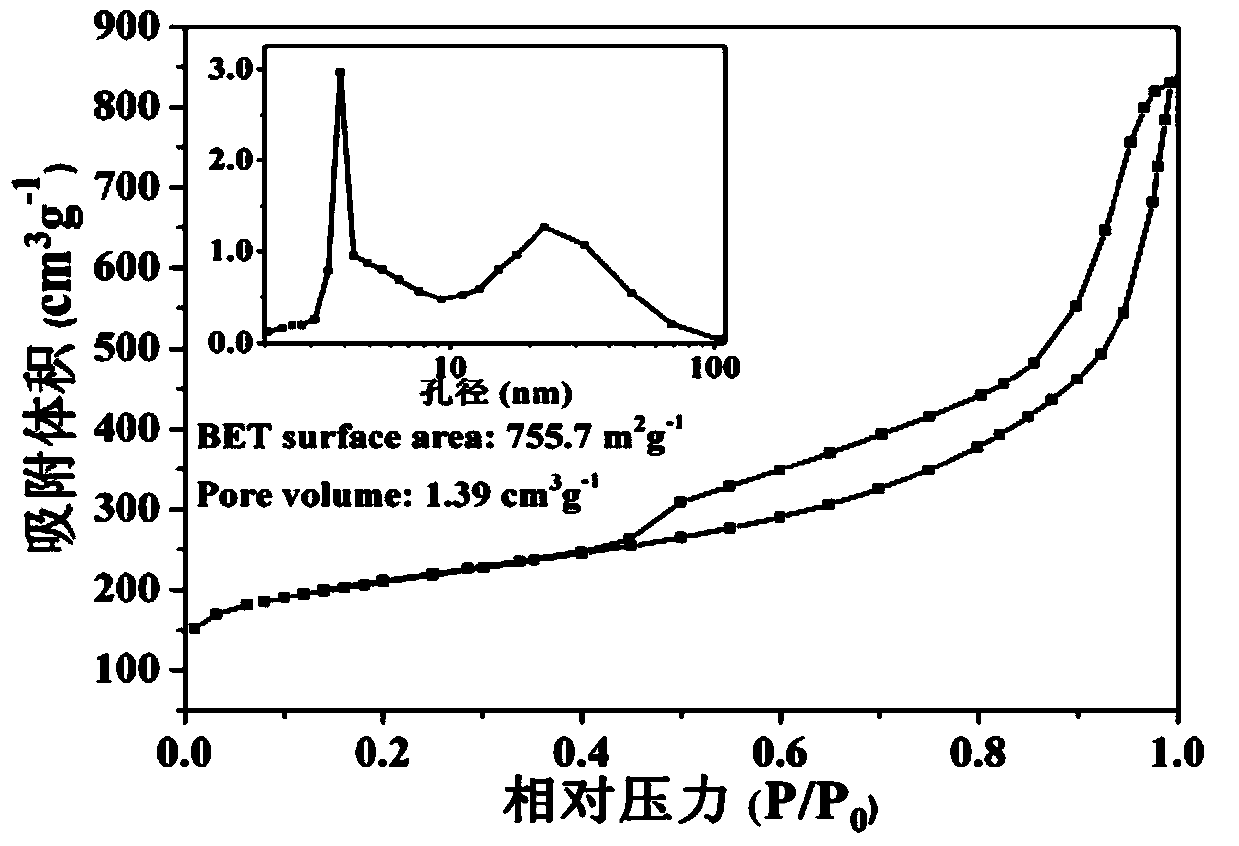
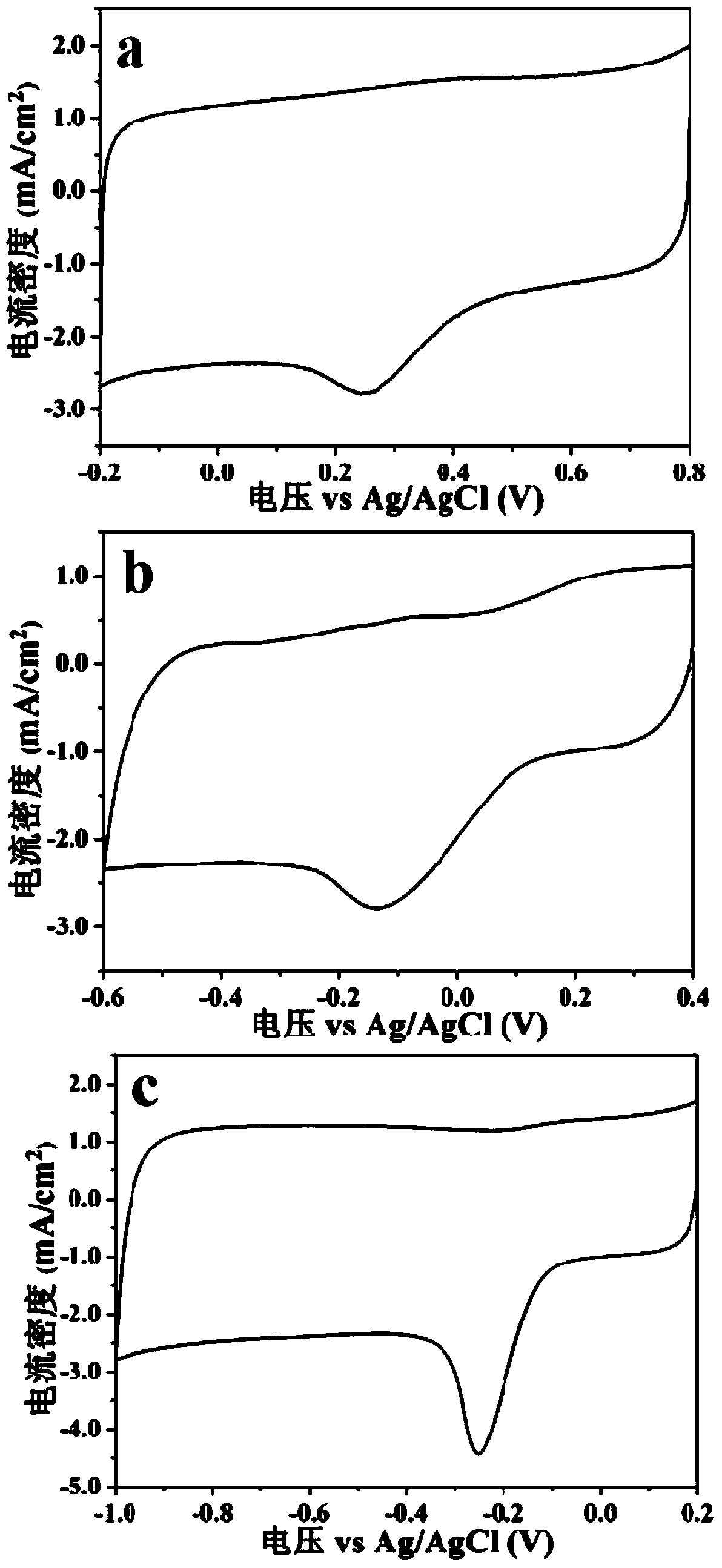
Nonmetal oxygen reduction catalyst and preparation method thereof
A catalyst, non-metal technology, applied in the field of oxygen reduction catalyst without metal element and its preparation, can solve the problems of complicated process conditions, carbon monoxide poisoning, catalyst oxidation and the like, achieve simple and safe preparation process, improve catalytic activity and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0020] The following are examples of the present invention, providing detailed implementation and specific operation process, the purpose of which is only to better understand the content of the present invention. The scope of protection of the invention is therefore not restricted by the examples given.
[0021] The test method of the catalyst performance that the present invention uses is as follows:
[0022] Mix 2 mg of the prepared nitrogen-phosphorous co-doped porous carbon material with 1 mL of 5wt% Nafion emulsion and ethanol (volume ratio 1:9) and sonicate for 30 minutes to obtain a dispersion; take 10 μL of the dispersion and apply it on a rotating circle After drying on the disc electrode at room temperature, a thin film electrode was obtained. Using a three-electrode system with Ag / AgCl electrode as the reference electrode and Pt wire as the counter electrode, in oxygen-saturated 0.1mol / L KOH, 0.1mol / L PBS, 0.5mol / L H 2 SO 4 Cyclic voltammetry and linear sweep vo...
Embodiment 1
[0024] The aqueous solution of polystyrene microspheres with a mass fraction of 10% and a particle size of 160 nm and dimethyl sulfoxide solution with 10% polyvinyl alcohol were uniformly mixed at a volume ratio of 1:1 at 70°C, and then the obtained The precursor solution was frozen at -20°C for 4 hours and thawed at 25°C for 1 hour to obtain a composite hydrogel of polyvinyl alcohol and polystyrene. 10 g of composite hydrogels were chemically cross-linked with 50 mL of 0.75% glutaraldehyde aqueous solution for 6 hours. The chemically crosslinked composite hydrogel was immersed in 10 mL of aqueous solution of cyanamide and phosphoric acid (the mass ratio of phosphoric acid to cyanamide was 0.2:1) for 12 hours to reach adsorption swelling equilibrium. Then dry at 80°C for 24 hours. The soaked and dried solid was pyrolyzed at 900° C. in an inert atmosphere and kept for 3 hours. Then naturally cooled to room temperature to obtain a hierarchically porous nitrogen-phosphorus co-d...
Embodiment 2
[0027] The aqueous solution of polystyrene microspheres with a mass fraction of 10% and a particle size of 160nm and the dimethyl sulfoxide solution of 10% polyvinyl alcohol were uniformly mixed at a volume ratio of 1:1 at 70°C, and then the obtained The precursor solution was frozen at -20°C for 4 hours and thawed at 25°C for 1 hour to obtain a composite hydrogel of polyvinyl alcohol and polystyrene. 10 g of composite hydrogels were chemically cross-linked with 50 mL of 0.75% glutaraldehyde aqueous solution for 6 hours. The chemically crosslinked composite hydrogel was soaked in 10 mL of 50% cyanamide aqueous solution for 12 hours to reach adsorption swelling equilibrium. Then dry at 80°C for 24 hours. The soaked and dried solid was pyrolyzed at 900° C. in an inert atmosphere and kept for 3 hours. Then cooled naturally to room temperature to obtain hierarchically porous nitrogen-doped carbon materials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Large hole diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com