Biquaternary ammonium salt-containing diamine or diol monomer, preparation method of monomer, water-based non-toxic antibacterial polyurethane emulsion prepared by monomer, and preparation method of emulsion
A technology of polyurethane emulsion and biquaternary ammonium salt, applied in the field of polyurethane emulsion, can solve the problems of weak cationic activity, limited application, cytotoxicity, etc., and achieve the effect of improving antibacterial effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] After the amine group of DAPA is protected by conventional tert-butoxy group, it is dissolved in dichloromethane with NG1 at a molar ratio of 1.1:1.0, and then the temperature is lowered to 0°C or below, and 1.1 molar amount of DAPA is added. Double compound condensing agent, in which N, N'-dicyclohexylcarbodiimide: 1-hydroxybenzotriazole = 1.05: 1, react at room temperature for 24 hours, and obtain bis-quaternary ammonium salt diamine protected by tert-butoxy Add the bis-quaternary ammonium salt diamine protected by tert-butoxy to dissolve in methanol, then add ethyl acetate with saturated hydrogen chloride equal to the volume of methanol at room temperature, stir for 3 hours, and then use C18-RP reverse-phase silica gel column chromatography The double quaternary ammonium salt diamine monomer was purified by the method, and the eluent was water / methanol.
Embodiment 2
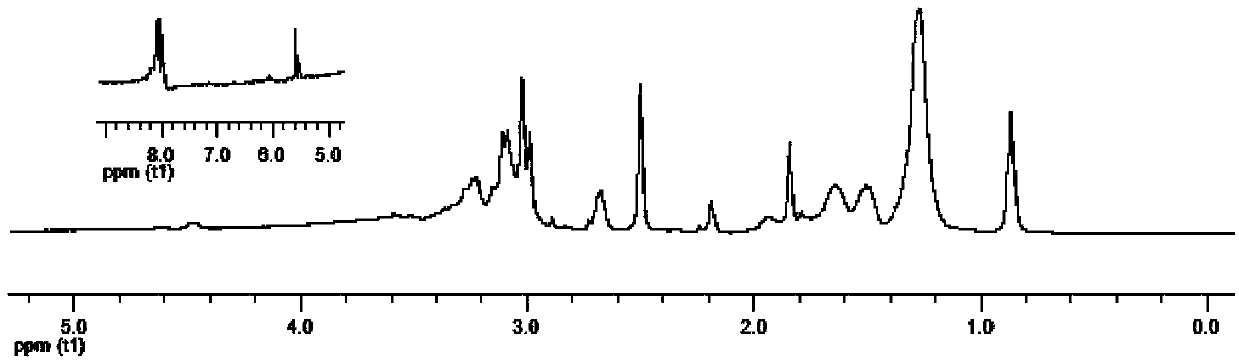
[0092] After the amine group of lysine is protected by conventional tert-butoxy group, it is dissolved in tetrahydrofuran with NG8 at a molar ratio of 1.05:1.0, and then the temperature is lowered to 0°C or below, and 1.05 times the molar amount of lysine is added Composite condensing agent, in which N, N'-dicyclohexylcarbodiimide: N-hydroxysuccinimide = 1.1: 1, react at room temperature for 36 hours to obtain bis-quaternary ammonium diamine protected by tert-butoxy; Butoxy-protected bis-quaternary ammonium diamine is dissolved in ethanol, then add ethyl acetate with saturated hydrogen chloride equal to the volume of ethanol at room temperature, stir for 3 hours, and purify by precipitation (methanol / petroleum ether) to obtain bis-quaternary ammonium salt diamine monomer. The proton nuclear magnetic resonance spectrum figure of this double quaternary ammonium salt diamine monomer is shown in figure 1 .
Embodiment 3
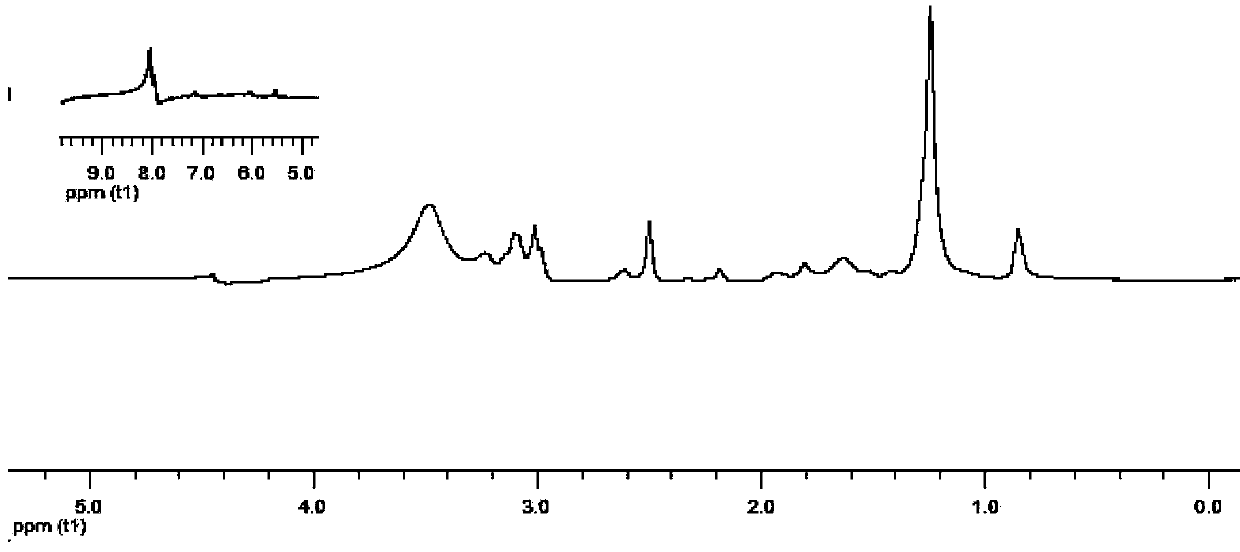
[0094] After the amine group of lysine is protected by conventional tert-butoxy group, it is dissolved in N,N dimethylformamide with NG12 at a molar ratio of 1.2:1.0, and then the temperature is lowered to 0°C or below, and lysine is added 1.2 times the molar weight of the compound condensing agent, where N, N'-dicyclohexylcarbodiimide:N-hydroxysuccinimide=1.3:1, reacted at room temperature for 36h, and obtained the biquaternary Ammonium salt diamine; add the bisquaternary ammonium salt diamine protected by tert-butoxy group into dichloromethane to dissolve, then add ethyl acetate with saturated hydrogen chloride equal to the volume of dichloromethane at room temperature, stir for 4 hours, and then use C18 -RP reverse-phase silica gel column chromatography to obtain bis-quaternary ammonium diamine monomer, and the eluent is water / methanol. The proton nuclear magnetic resonance spectrum figure of this double quaternary ammonium salt diamine monomer is shown in figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com