Amino acid base biodegradable 2-expoxy compound and preparation method thereof
An amino acid-based, diepoxy-based technology, applied in organic chemistry, bulk chemical production, etc., can solve problems such as lack of biocompatibility and degradability, and achieve good biodegradation, biocompatibility, and high storage stability , The preparation method is simple and efficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
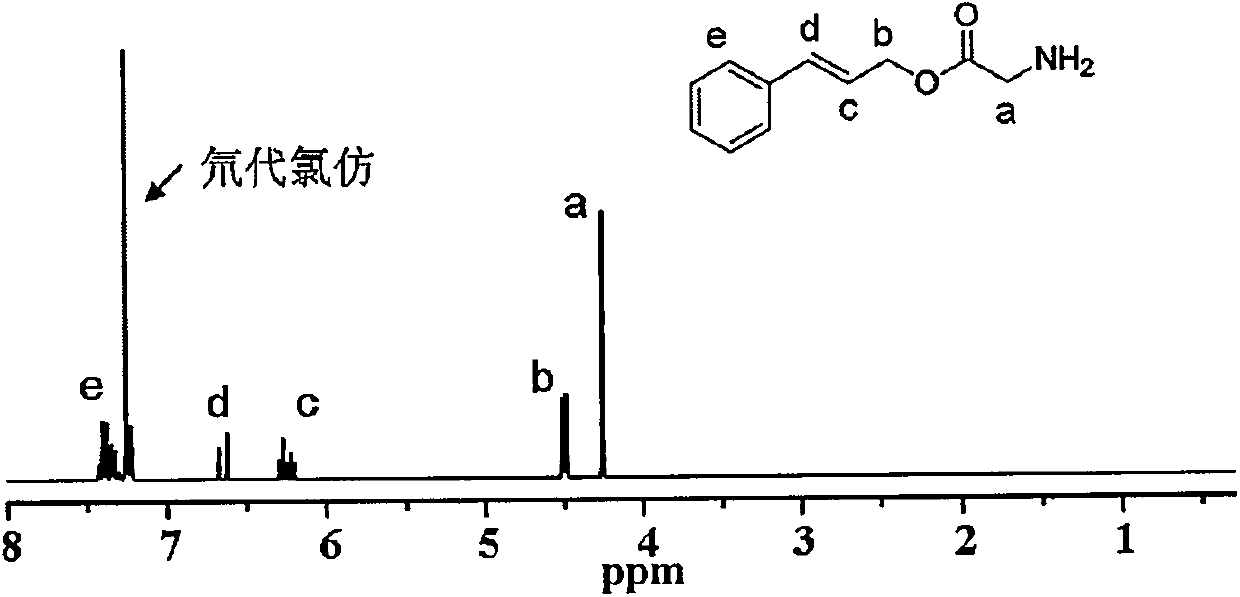
[0027] 1. Take a round-bottomed flask with a magnet and place it in an ice bath, add 1.5g of glycine and 30mL of deionized water in sequence, and then add 30mL of 1mol / L sodium bicarbonate aqueous solution; weigh 7.28g of di-tert-butyl dicarbonate, After dissolving with 35mL of tetrahydrofuran, it was slowly added to a round bottom flask under stirring, and the mixture was reacted at room temperature for 24 hours. After the reaction was completed, it was repeatedly extracted with diethyl ether, and the obtained supernatant was dried with a large amount of anhydrous sodium sulfate, and then filtered with a G4 sand core funnel, and the filtrate was dried to obtain the product tert-butoxycarbonyl-protected amino group glycine.
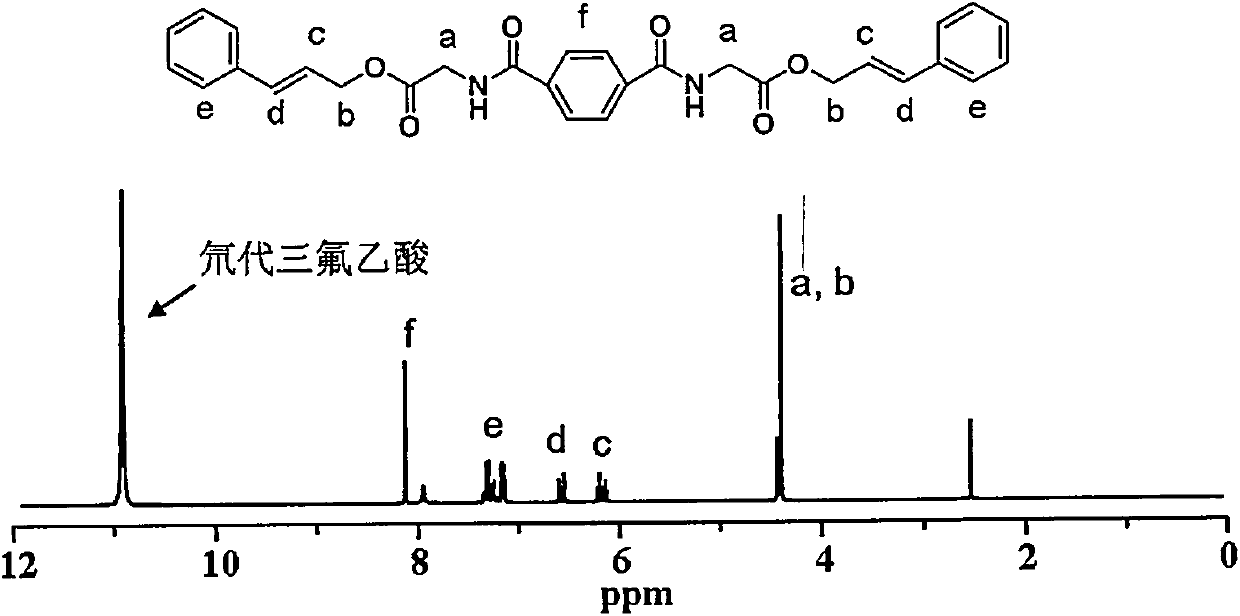
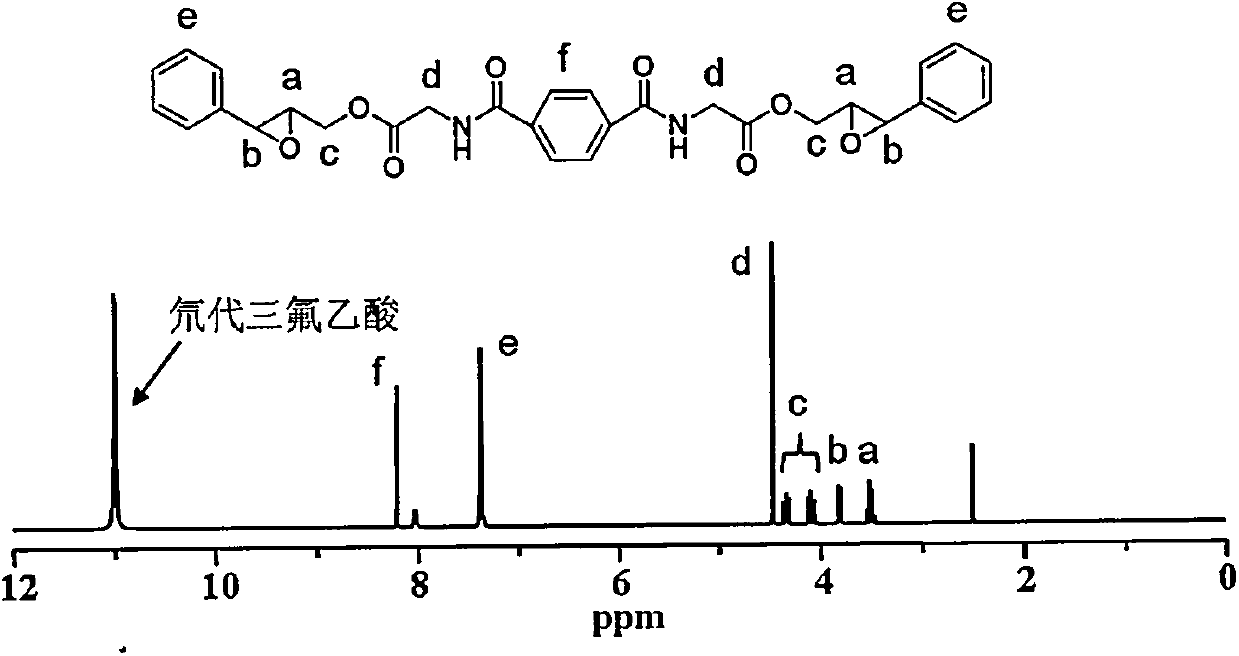
[0028] 2. Dissolve 1.75g of tert-butoxycarbonyl-protected amino glycine and 1.34g of cinnamyl alcohol in dichloromethane, and then add 9.55g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene After the reaction of amine hydrochloride and 1.22g of 4-dim...
Embodiment 2
[0034] 1. Take a round-bottomed flask with a magnet and place it in an ice bath, add 1.78g of alanine and 30mL of deionized water in sequence, and then add 30mL of 1mol / L sodium bicarbonate aqueous solution; weigh 7.28g of di-tert-butyl dicarbonate After dissolving the ester with 35mL of tetrahydrofuran, it was slowly added to the round bottom flask under stirring, and the mixture was reacted at room temperature for 24 hours. After the reaction was completed, it was repeatedly extracted with diethyl ether, and the obtained supernatant was dried with a large amount of anhydrous sodium sulfate, and then filtered with a G4 sand core funnel, and the filtrate was dried to obtain the product tert-butoxycarbonyl-protected amino-alanine.
[0035] 2. Dissolve 1.89g of alanine and 1.34g of cinnamyl alcohol in dichloromethane, and then add 9.55g of 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride and 1.22g of 4-dimethylaminopyridine were washed with water and dried several ...
Embodiment 3
[0041] 1. Take a round-bottomed flask with magnets and place it in an ice bath, add 2.34g of valine and 30mL of deionized water in sequence, and then add 30mL of 1mol / L sodium bicarbonate aqueous solution; weigh 7.28g of di-tert-butyl dicarbonate After dissolving the ester with 35mL of tetrahydrofuran, it was slowly added to the round bottom flask under stirring, and the mixture was reacted at room temperature for 24 hours. After the reaction was completed, it was repeatedly extracted with diethyl ether, and the obtained supernatant was dried with a large amount of anhydrous sodium sulfate, and then filtered with a G4 sand core funnel, and the filtrate was dried to obtain the product valine with tert-butoxycarbonyl-protected amino group.
[0042] 2. Dissolve 2.17g of valine and 1.34g of cinnamyl alcohol in dichloromethane, and then add 9.55g of 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride and 1.22g of 4-dimethylaminopyridine were washed with water and dried sev...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap