A high-rate aqueous alkaline metal electrochemical cell positive electrode material and preparation method thereof
A technology of battery positive electrode and positive electrode material, which is applied to battery electrodes, alkaline storage batteries, circuits, etc., can solve the problems of rapid capacity decay and low effective recyclable specific capacity, and achieves simple process lines, easy industrialized continuous production, and low cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
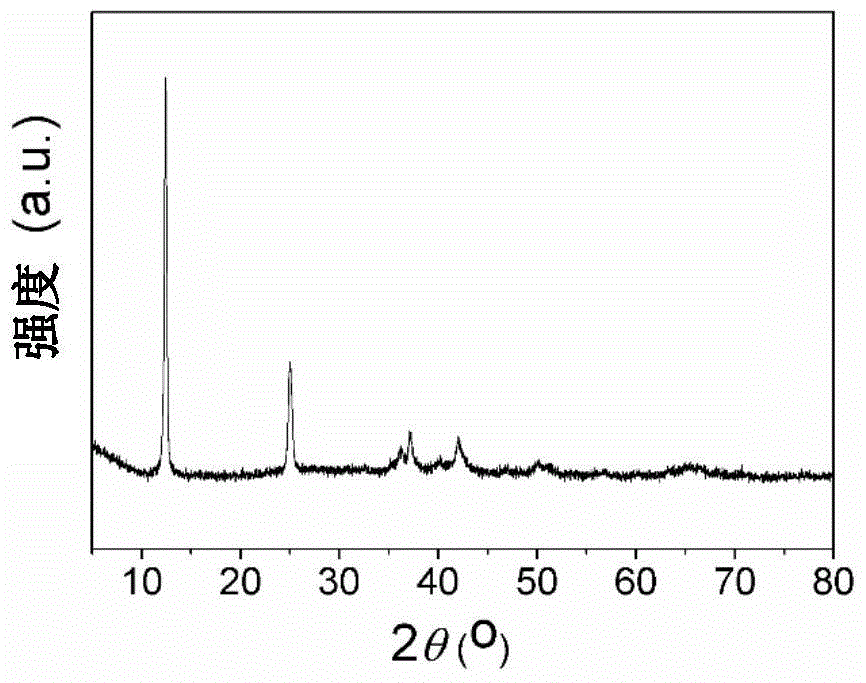
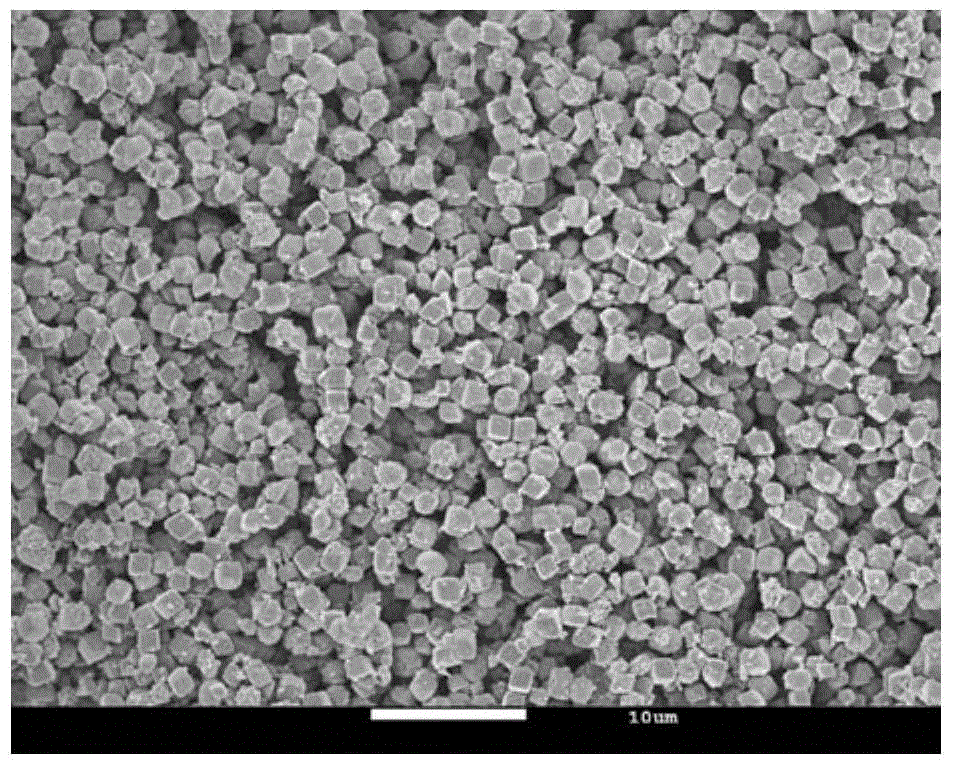
[0038] Put 1.3g of potassium carbonate and 2.3g of manganese carbonate in a 500mL beaker, add 200mL of ethanol, stir at 50℃ to dryness, grind in an agate mortar for 20min, then sinter in an air furnace at 550℃ for 8h, wash three times with water, wash three times with alcohol, and dry. . The layered K is prepared 0.27 MnO 2 (see figure 1 , 2 ).
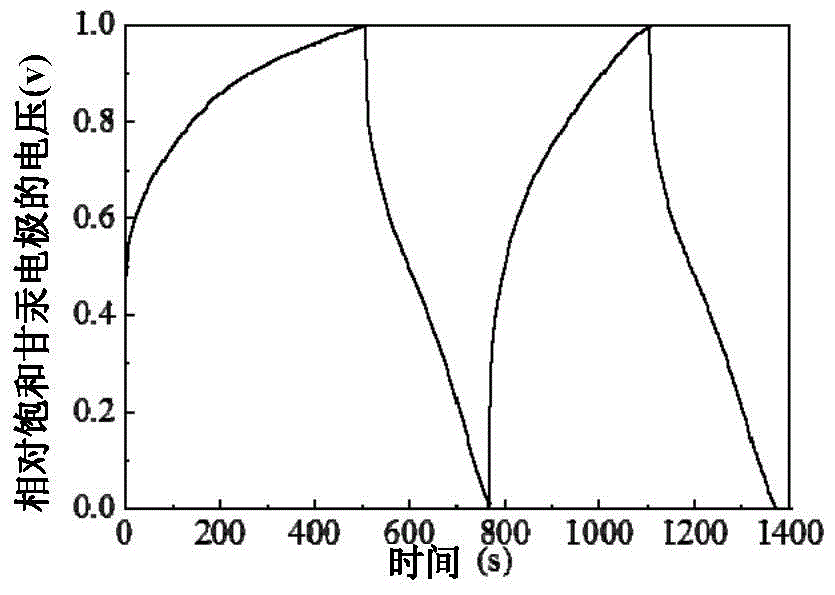
[0039] Using the K prepared in Example 1 0.27 MnO 2 , The conductive carbon black and the binder polyvinylidene fluoride are mixed in a mass ratio of 80:10:10, and N-methylpyrrolidone is used as a solvent, coated on the stainless steel mesh, and vacuum dried for 12 hours. Then use 1M sodium sulfate as the electrolyte, activated carbon as the counter electrode, and saturated calomel as the reference electrode for the constant current (100mA / g) charge and discharge test (such as image 3 ).
Embodiment 2
[0041] The flower-like layered manganese dioxide was prepared by microwave hydrothermal for 10 minutes, which has a nanometer morphology, and then 0.17 g of layered manganese dioxide and 0.13 g of potassium carbonate were placed in a 100 mL beaker, adding 20 mL of ethanol, and stirring at 50°C to Dry, grind in an agate mortar for 20 minutes, then sinter in an air furnace at 500°C for 10 hours, wash three times with water, wash three times with alcohol, and dry. The layered K is prepared 0.27 MnO 2 (see Figure 4 , 5 ).
[0042] Using the K prepared in Example 2 0.27 MnO 2 , The conductive carbon black and the binder polyvinylidene fluoride are mixed in a mass ratio of 80:10:10, and N-methylpyrrolidone is used as a solvent, coated on the stainless steel mesh, and vacuum dried for 12 hours. Then use 1M sodium sulfate as the electrolyte, activated carbon as the counter electrode, and saturated calomel as the reference electrode for the CV test. The scanning speed is 1mV.s -1 (See I...
Embodiment 3
[0044] Put 3g potassium carbonate and 4g manganese trioxide in a ball mill tank, add appropriate amount of acetone, ball mill with a planetary ball mill for 8 hours, dry the sample at 50°C, then sinter it in an air furnace at 700°C for 16 hours, wash three times with water, and wash three times with alcohol. Dry treatment. The layered K is prepared 0.125 MnO 2 (see Picture 9 ).
[0045] Using K prepared in Example 3 0.125 MnO 2 , The conductive carbon black and the binder polyvinylidene fluoride are mixed in a mass ratio of 80:10:10, and N-methylpyrrolidone is used as a solvent, coated on the stainless steel mesh, and vacuum dried for 12 hours. Then use 1M sodium sulfate as the electrolyte, activated carbon as the counter electrode and saturated calomel as the reference electrode for CV (scanning speed is 1mV.s) -1 ) And constant current charge and discharge test, current intensity 10mA / g (such as Picture 10 , Picture 11 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com