Gel polymer electrolyte containing natural high molecular material as well as preparation method and application thereof
A gel polymer, natural polymer technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of "white" pollution and poor biocompatibility, and achieve good compatibility and biocompatibility. The effect of good performance, cycle performance and rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Under stirring at room temperature, dissolve 50 mg of methyl cellulose with a methyl content of about 30% in 20 mL of deionized water, and then cast it on a glass plate. Moisture was evaporated at 80°C, followed by vacuum drying at 100°C to obtain a film with a thickness of 20 µm.
[0024] (2) Transfer the above-mentioned methylcellulose film into a glove box at room temperature, soak it in 1M LiPF6 electrolyte (model LIB315, purchased from Zhangjiagang Guotai Huarong Chemical New Material Co., Ltd.) for 12 hours to obtain the gel polymer electrolyte.
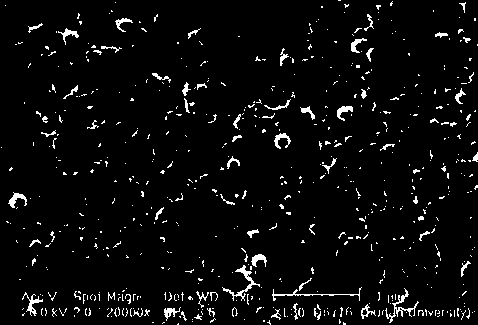
[0025] The methylcellulose film obtained by the above method is measured: the liquid absorption rate of the electrolyte, the ionic conductivity after absorbing the electrolyte, the lithium ion transfer coefficient and the temperature at which the electrolyte is volatilized. The results are shown in Table 1, and the scanning Electron microscopy was used to analyze the morphology, and the results are shown in figure ...
Embodiment 2
[0041](1) Under stirring at room temperature, dissolve 10 g of carboxymethylcellulose (molecular weight: 700,000, carboxymethyl ratio: 0.9) in 40 L of deionized water, and add 2.5 L of N,N-dimethylformamide, and then Cast on a glass plate. Moisture was evaporated at 80°C, and then vacuum-dried at 100°C to obtain a film with a thickness of 30 µm.
[0042] (2) Transfer the above-mentioned carboxymethyl cellulose film into a glove box at room temperature, soak it in 1M LiPF6 electrolyte (model LIB315, purchased from Zhangjiagang Guotai Huarong Chemical New Material Co., Ltd.) for 12 hours to obtain gel polymerization matter electrolyte.
[0043] The carboxymethyl cellulose film obtained by the above method is measured according to Example 1: the liquid absorption rate of the electrolyte, the ion conductivity after absorbing the electrolyte, the lithium ion transfer coefficient and the temperature at which the electrolyte is volatilized, the results are shown in the table 1, and...
Embodiment 3
[0045] (1) Under stirring at room temperature, dissolve 50 mg of soluble starch in 20 mL of deionized water, and then cast it on a glass plate. Moisture was evaporated at 80°C, followed by vacuum drying at 100°C to obtain a film with a thickness of 20 µm.
[0046] (2) Fix the soluble starch film on the surface of smooth and clean aluminum foil, deposit the N-methylpyrrolidone mixture containing 10wt.%PVDF and 1wt.% silicon oxide on the soluble starch film by electrospinning, and place it at 80 ℃ heating plate to volatilize the solvent, and then deposit a layer of PVDF containing silicon oxide on the other side to obtain a composite film of soluble starch and PVDF.
[0047] (3) According to the method of step (2) in Example 1 or 2, after cutting the composite film of soluble starch and PVDF into an appropriate size, place it in a vacuum drying oven at 80° C. for 24 hours to dry and remove trace solvents, and vacuum cool Transfer to a glove box at room temperature. Soak the co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com