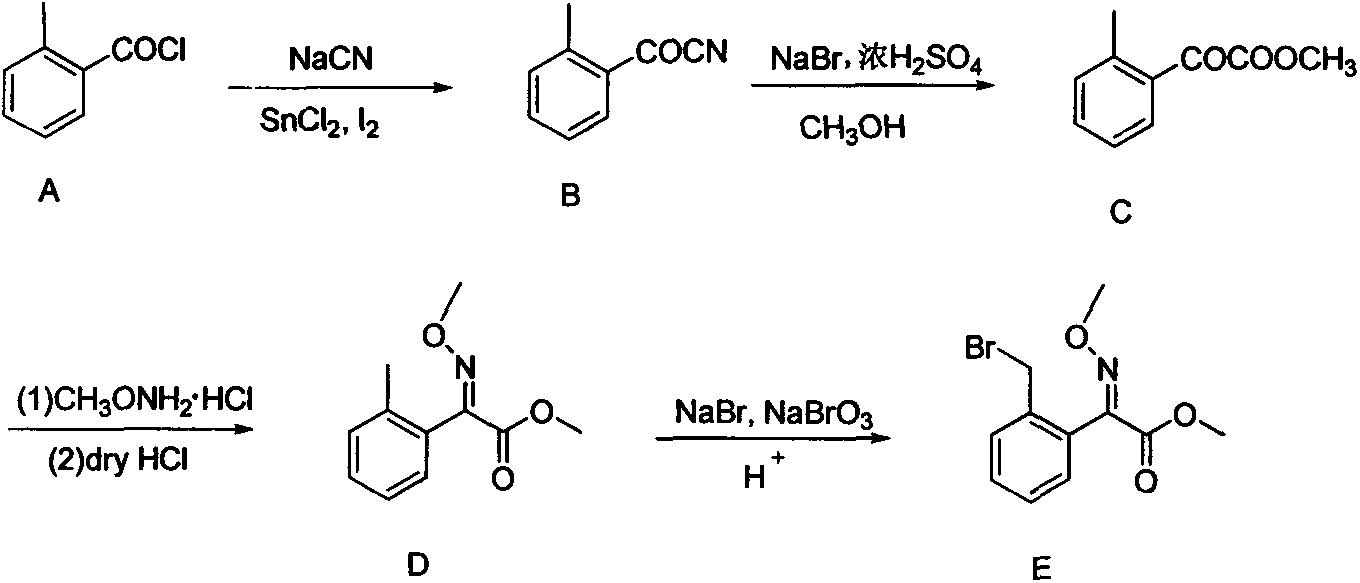
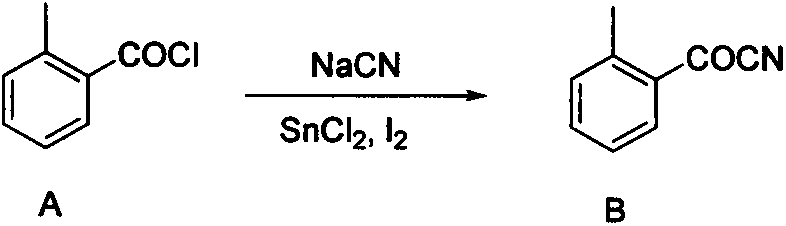
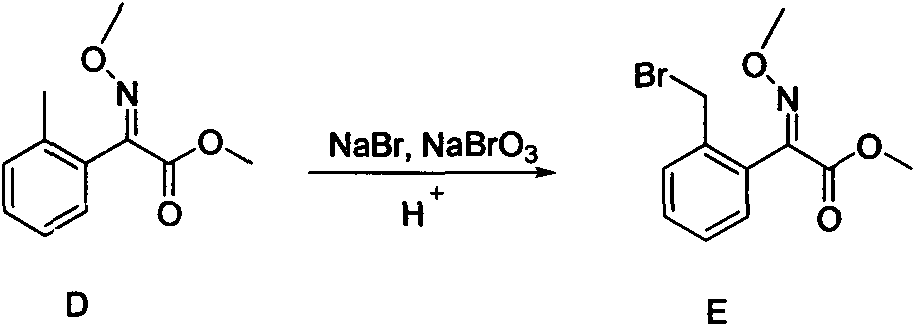Preparation method of improved 2-(2'-bromomethylphenyl)-2-carbonyl methyl acetate
A kind of technology of methyl carbonyl acetate and bromomethyl phenyl, applied in the field of preparation of 2-(2'-bromomethyl phenyl-2-methyl carbonyl acetate, it can solve the problem of rising cost, polluting the environment and wasting energy and other problems, to achieve the effect of improving the reaction method and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 500ml round bottom flask, add A27.9g (180mmol), sodium cyanide 9.3g (190mmol), tetrabutylammonium bromide 1.5g (4.6mmol), tin dichloride 0.11g (0.6mmol), iodine 0.15g (0.6mmol) and toluene 200ml. The system was heated up to 70° C., and reacted at this temperature for 4 hours, and the conversion rate of the raw material was about 65% according to gas chromatography of the reaction liquid. At this time, the system was lowered to room temperature (20° C.), and 50 ml of aqueous sodium hydroxide solution with pH=10 was added to the reaction bottle, and the stirring reaction was continued for 1 hour. TLC detected that the reaction was complete. The system was neutralized with 5% aqueous sodium bicarbonate solution, filtered, separated, the aqueous phase was extracted twice with 50 ml of toluene, and the organic phases were combined and dried. 24.2 g of the crude product was obtained by precipitation, the content was 79.3%, and the yield was 73%.
Embodiment 2
[0023] In a 500ml round bottom flask, add A27.9g (180mmol), sodium cyanide 9.3g (190mmol), tetrabutylammonium bromide 1.5g (4.6mmol), tin dichloride 0.11g (0.6mmol), iodine 0.15g (0.6mmol) and toluene 200ml. The system was heated up to 80° C., and reacted at this temperature for 4 hours, and the conversion rate of the raw material was about 75% according to gas chromatography of the reaction liquid. At this time, the system was lowered to room temperature (20° C.), and 50 ml of aqueous sodium hydroxide solution with pH=10 was added to the reaction bottle, and the stirring reaction was continued for 1 hour. TLC detected that the reaction was complete. The system was neutralized with 5% aqueous sodium bicarbonate solution, filtered, separated, the aqueous phase was extracted twice with 50 ml of toluene, and the organic phases were combined and dried. 23.7 g of the crude product was obtained by precipitation, the content was 88.7%, and the yield was 80%.
Embodiment 3
[0025] A27.9g (180mmol), sodium cyanide 9.3g (190mmol), tetrabutylammonium bromide 1.5g (4.6mmol) and toluene 200ml were added in a 500ml round bottom flask. The system was heated up to 80° C., and reacted at this temperature for 4 hours, and the conversion rate of the raw material was about 45% according to gas chromatography of the reaction liquid. At this time, the system was lowered to room temperature (20° C.), and 50 ml of aqueous sodium hydroxide solution with pH=10 was added to the reaction flask, and the stirring reaction was continued for 1 hour. TLC detected that the reaction was complete. The system was neutralized with 5% aqueous sodium bicarbonate solution, filtered, separated, the aqueous phase was extracted twice with 50 ml of toluene, and the organic phases were combined and dried. 22.9 g of the crude product was obtained by precipitation, the content was 60%, and the yield was 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com