Matrine diatomic alcohol plastid temperature-sensitive gel and preparation method thereof
A temperature-sensitive gel and matrine technology, applied in liquid delivery, pharmaceutical formulations, aerosol delivery, etc., can solve the limitations of the clinical application of matrine alkaloid thermo-sensitive gel, short local action time, and patient Poor compliance and other problems, to achieve the effect of being conducive to industrial production and popularization, improving the therapeutic effect and prolonging the effect time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
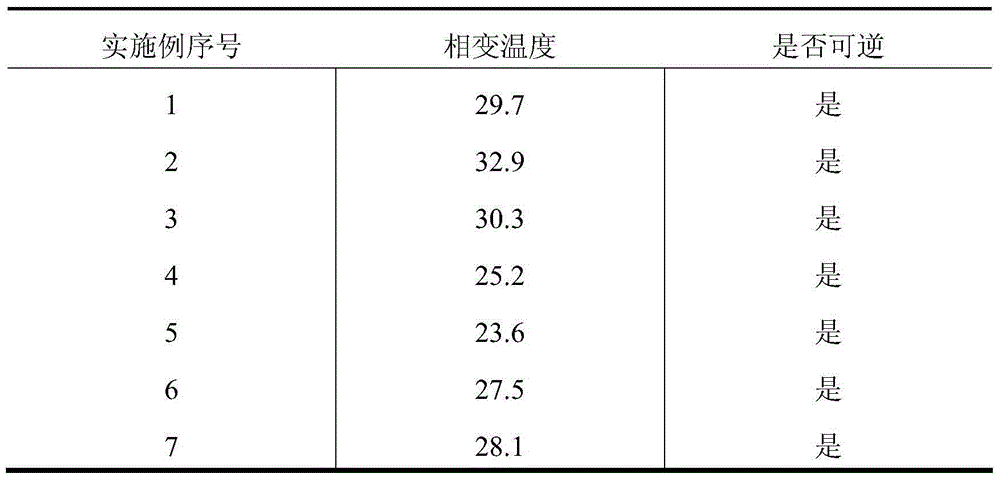
Examples
Embodiment 1
[0032] 1) Dissolve 0.25g of matrine in 53.65g of phosphate buffer (pH 4.5), keep a constant temperature of 37±1°C; slowly add lecithin low molecular weight alcohol solution under stirring (lecithin 1.5g, low molecular weight Alcohol 13.0g, ethanol: propylene glycol ratio of 1:1); under ice bath conditions, use a probe-type ultrasonic instrument to ultrasonically disperse for 90S, and filter with a 0.45μm filter membrane to obtain a whey-colored matrine glycol plastid suspension;
[0033] 2) Add 0.6g chitosan to matrine diol plastid suspension, store at low temperature (-4°C, 24h); then add 6g glycerol and 25g poloxamer (poloxamer 407: poloxamer Sham 188 (4:1), stir evenly and store at low temperature (-4°C, 24h), to obtain whey-colored matrine diol body temperature-sensitive gel.
[0034] 10g of each unit preparation is divided into disposable vaginal injectors, packaged, and injected into the vagina during use.
Embodiment 2
[0036] 1) Dissolve 1.0g of matrine in 41.2g of phosphate buffer (pH 4.0), keep a constant temperature of 37±1°C; slowly add lecithin low molecular weight alcohol solution under stirring (lecithin 1.0g, low molecular weight Alcohol 30.0g, ethanol: propylene glycol ratio of 2:1); under ice bath conditions, use a probe-type ultrasonic instrument to ultrasonically disperse for 90S, and filter with a 0.45μm filter membrane to obtain a whey-colored matrine glycol plastid suspension;
[0037] 2) Add 0.8g chitosan to matrine diol plastid suspension, store at low temperature (-10°C, 48h); then add 10g glycerol and 16g poloxamer (poloxamer 407: poloxamer Sharm 188 (3:1), stir evenly and store at low temperature (-4°C, 24h), to obtain whey-colored matrine diol body temperature-sensitive gel.
[0038] Each unit preparation is 4g, divided into disposable vaginal injectors, packaged, and injected into the vagina during use.
Embodiment 3
[0040] 1) Dissolve 0.25g of matrine in 55g of phosphate buffer (pH 3.5), keep a constant temperature of 37±1°C; slowly add lecithin low molecular alcohol solution under stirring (lecithin 0.25g, low molecular alcohol 18.5g, ethanol: propylene glycol ratio of 5:1); under ice bath conditions, use a probe-type ultrasonic instrument to ultrasonically disperse for 90S, and filter through a 0.45μm filter membrane to obtain a whey-colored matrine glycol plastosome suspension;
[0041]2) Add 1.0g chitosan to matrine diol plastid suspension, store at low temperature (-4°C, 24h); then add 5g glycerol and 20g poloxamer (poloxamer 407: poloxamer Sharm 188 (1:1), stir evenly and store at low temperature (-10°C, 48h), to obtain whey-colored matrine diol body temperature-sensitive gel.
[0042] 10g of each unit preparation is divided into disposable vaginal injectors, packaged, and injected into the vagina during use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com