Preparation method of high-purity lithium hexafluorophosphate
A technology of lithium hexafluorophosphate and hexafluorophosphate, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of few production links, many production links, and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
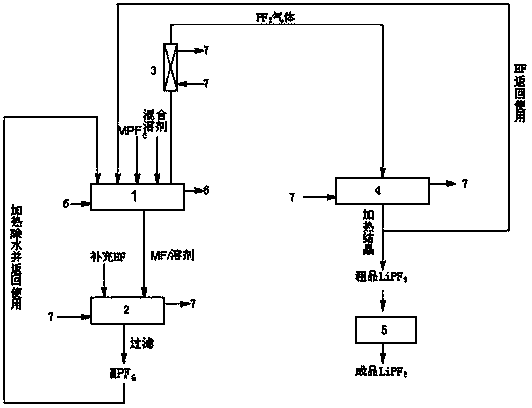
Image
Examples
Embodiment 1
[0032] Take 9.26g of LiF and 60.64g of anhydrous HF to prepare a lithium fluoride solution to form an absorption tank; at the same time, take 60.07g of potassium hexafluorophosphate and place it in a solution composed of 401.21g of hydrogen fluoride and 80.02g of trifluoromethanesulfonic acid In the process, a reaction tank is formed, and the two are connected by a polytetrafluoroethylene hose to form a closed system. After the closed system is formed, the reaction tank is heated, and the heating is slow heating, and the temperature is maintained above 80°C for 8 hours until the weight of the absorption tank does not change. After the reaction is over, lithium hexafluorophosphate is obtained by evaporation and crystallization in the absorption tank. The mass of LiF added is 9.26g, if all of it is converted into LiPF 6 The mass is 54.14g, and LiPF is obtained after drying 6 The mass is 53.67g, so the potassium hexafluorophosphate therein is considered to be completely converte...
Embodiment 2
[0036] Take 18.25g of LiF and 90.3g of anhydrous HF to prepare a lithium fluoride solution to form an absorption tank; at the same time, take 121.5g of potassium hexafluorophosphate and place it in a solution composed of 525g of hydrogen fluoride and 128g of trifluoromethanesulfonic acid. A reaction tank is formed, and the two are connected by a polytetrafluoroethylene hose to form a closed system. Then, the reaction tank is heated slowly, and at the same time, the temperature is maintained above 80°C for 8 hours until the weight of the absorption tank no longer changes. 121.5gKPF added during the test 6 If all decomposed, the available LiPF 6 The mass of 100.4g. After the end of the test, after evaporation and crystallization obtained in the absorption tank, LiPF was obtained. 6 The total mass of is 100.02g, so the potassium hexafluorophosphate therein is considered to be completely converted.
[0037] Table II
[0038] project
Embodiment 3
[0040] After the reaction tank was drained, the weight of the solid wet material was 176.84g, and 100g of acetone was added to remove trifluoromethanesulfonic acid, and the weight of the wet material was 120.32g. According to the complete decomposition of potassium hexafluorophosphate, 60.28g of phosphoric acid was added, and an excess of HF was added. The mass of HF is 321.27g, after stirring for 24 hours, it is filtered, and the resulting solid is sucked dry to obtain 134.24g of white crystals, which are then transferred to a vacuum oven for drying. The drying conditions are: 60°C, vacuum drying for 6 hours, Replace with nitrogen once every half hour, then raise the temperature to 190° C., and after drying for 24 hours, take out the material to obtain 107.2 g of solid material. The above experiments were repeated with the same experimental conditions. It can be seen from the experimental process that if the amount of LiF is assumed to remain unchanged during the KF recycling...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com