Preparation method of 1, 3-dimethyl-pentylaminehydrochloride
A technology of dimethylpentylamine hydrochloride and methyl group, which is applied in the field of organic synthesis and preparation chemistry, can solve the problems of high industrialization cost, poor product purity, and many process steps, and achieves low production cost, simple operation and controllability. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
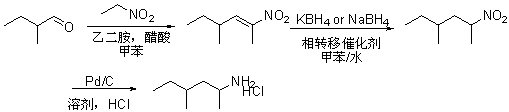
[0023] A kind of preparation method of 1,3-dimethylamylamine hydrochloride, the reaction process schematic diagram of its preparation method is as figure 1 shown, namely
[0024] Firstly, using 2-methylbutyraldehyde as the starting material, in toluene solvent, the ethylenediamine and acetic acid composite catalyst are used to catalyze the condensation reaction of 2-methylbutyraldehyde and nitroethane to prepare nitroolefin compounds;
[0025] Then, the resulting nitroalkene compound is subjected to an alkene reduction reaction by potassium borohydride or sodium borohydride in a mixed solvent composed of toluene and water under the condition of a phase transfer catalyst to obtain 4-methyl 2-nitro Hexane;
[0026] Then, the obtained 4-methyl 2-nitrohexane, in an alcohol solvent, is hydrogenated and reduced to an amino group by palladium carbon, and then salified by hydrochloric acid to obtain 1,3-dimethylpentylamine hydrochloride Crude salt;
[0027] Finally, the obtained cr...
Embodiment 1
[0029] A kind of preparation method of 1,3-dimethylamylamine hydrochloride, specifically comprises the steps:
[0030] (1) In a 500mL reaction bottle, add 165g of solvent 1, add 3.4g of ethylenediamine, slowly add 6.8g of acetic acid dropwise under stirring, then dropwise add 70g of 2-methylbutyraldehyde, and then slowly dropwise add nitro Ethane 67g, control the temperature at 84-88°C to reflux with water to react for 8-10h, after the reaction is completed, cool to below 35°C, add 50ml of water, stir for static separation, remove the water layer, and the obtained toluene-containing organic The phase is the crude product of nitroolefin compound;
[0031] Described solvent 1 is toluene;
[0032] In the process of condensation and dehydration of 2-methylbutyraldehyde and nitroethane in the above steps, calculated by molar ratio, that is, 2-methylbutanal: nitroethane: composite catalyst is 1:1.0:0.1;
[0033] (2) Pour the crude nitroolefin compound obtained in step (1) into a 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com